Abstract
Wiskott Aldrich syndrome (WAS) is caused by mutations in the WAS gene that encodes for a protein (WASp) involved in cytoskeleton organization in hematopoietic cells. Several distinctive abnormalities of T, B, and natural killer lymphocytes; dendritic cells; and phagocytes have been found in WASp-deficient patients and mice; however, the in vivo consequence of WASp deficiency within individual blood cell lineages has not been definitively evaluated. By conditional gene deletion we have generated mice with selective deficiency of WASp in the B-cell lineage (B/WcKO mice). We show that this is sufficient to cause a severe reduction of marginal zone B cells and inability to respond to type II T-independent Ags, thereby recapitulating phenotypic features of complete WASp deficiency. In addition, B/WcKO mice showed prominent signs of B-cell dysregulation, as indicated by an increase in serum IgM levels, expansion of germinal center B cells and plasma cells, and elevated autoantibody production. These findings are accompanied by hyperproliferation of WASp-deficient follicular and germinal center B cells in heterozygous B/WcKO mice in vivo and excessive differentiation of WASp-deficient B cells into class-switched plasmablasts in vitro, suggesting that WASp-dependent B cell–intrinsic mechanisms critically contribute to WAS-associated autoimmunity.
Introduction
Wiskott-Aldrich syndrome (WAS) is a rare X-linked immunodeficiency caused by mutations of the WAS gene that is widely expressed within hematopoietic cells.1 The clinical phenotype of WAS is characterized by congenital thrombocytopenia, combined immunodeficiency, and eczema.1 The WAS protein (WASp) includes several functional domains that couple signal transduction to reorganization of the actin cytoskeleton. As a result, WASp has significant influence on processes such as cell adhesion, migration, assembly/turnover of cell surface receptors, and immunologic synapse formation.1,2 Several studies in patients with WAS and in Was knock-out (WKO) mice have shown that WASp plays a critical role in the function of T and natural killer lymphocytes and dendritic cells.1,3 However, the importance of WASp in B-cell development and function is less clearly defined. In vitro studies have shown that WASp-deficient B cells display defective actin polymerization on activation,4 and impaired migration in response to CXCL135 ; however, calcium mobilization and proliferation after B-cell receptor ligation were found to be normal or only slightly reduced.3 Studies in heterozygous Was+/− mice have found progressive in vivo selection for WASp-expressing cells in T, B, and natural killer lineages.6 Within the B-cell lineage, such selective advantage was especially prominent in marginal zone (MZ) B cells.2,6 However, the in vivo effect of selective deficiency of WASp expression within a single lineage has not been analyzed so far and is of critical importance to understand WAS pathophysiology. Recently, with the use of a chimeric BM transplantation reconstitution model, Becker-Herman et al have provided evidence that lack of WASp expression in B lymphocytes causes immune dysregulation and may lead to fatal autoimmunity.7 However, mixed chimerism in non-B lineages, irradiation-induced load of apoptotic bodies, and homeostatic B-cell proliferation may also have contributed to autoimmunity in that model.
We describe here the generation of mice in which the Was locus has been floxed by homologous recombination. By crossing these mice to mb1-Cre knock-in mice,8 which express the Cre recombinase under control of the CD79a promoter, the Was locus is selectively and efficiently deleted in B cells only, allowing analysis of the effect of B cell–restricted deficiency of WASp in vivo.
Methods
Mice
All mice were bred on a C57BL/6 background. WKO mice have been described.3 Mb1-Cre mice8 were a generous gift from Dr Michael Reth (Max Planck Institute of Immunobiology, Freiburg, Germany). The generation of B/WcKO is described in detail in the main text. The B/WcKO mouse colony was maintained by breeding Wasfl/fl females to Wasfl/ymb1+/Cre, resulting in generation of B/WcKO mice and of Wasfl/fl littermates that are phenotypically wild-type (WT). Unless otherwise stated, mice were examined at 6-10 weeks of age, and within an individual experiment WT, WKO, and B/WcKO mice were matched for age. All mouse experiments were approved by the institutional review board at Children's Hospital Boston.
FACS analysis of splenic and BM B-cell subpopulations
Supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article), lists the combinations of cell-surface markers and the sources of the reagents that were used to identify the various B-cell subpopulations in the BM, spleen, and lymph nodes. The polyclonal rabbit anti-WASp Ab used for FACS analysis has been previously described.6 Staining for WASp was performed with Fix and Perm permeabilization kit (BD Biosciences) followed by detection with allophycocyanin (APC)–labeled anti–rabbit IgG Fab fragment (Jackson ImmunoResearch Laboratories). Polyclonal rabbit serum IgG was used as control to define WASp+ versus WASp− populations. Trinitrophenyl (TNP)–specific B cells were detected by staining CD19+ splenic lymphocytes with a PE-labeled nitrophenyl (NP) hapten (Biosearch Technologies). Apoptosis of germinal center (GC) B cells was assessed by staining with APC-labeled annexin V (eBioscience), followed by flow cytometric analysis.
Generation of plasmablasts in vitro by stimulation with CpG
Splenocytes (2 × 105) of WT, B/WcKO, and WKO mice were plated in 96-well round-bottom plates in RPMI medium with 10% FCS and stimulated with 1.25μM CpG (ODN 1826; Invivogen). Five days later, class-switched plasmablasts were identified by flow cytometric expression of CD19 and intracellular IgG (combination of IgG1, IgG2a, and IgG2b Abs; BioLegend).
Immunofluorescence
Spleens from mice were frozen in OCT medium (Sakura Finetek) and 8- to 10-μm thin sections were cut in a cryostat microtome. After overnight incubation at room temperature, the slides were fixed in ice-cold acetone and blocked with 5% goat serum (Dako North America) and with avidin/biotin blocking kit (Vector Laboratories) in PBS. The slides were incubated with primary Abs for 30 minutes at room temperature, washed with PBS, incubated at room temperature for 30 minutes with secondary Ab, and washed again with PBS. The following reagents were used: biotinylated CD1d and APC-conjugated anti-B220 (BioLegend), streptavidin-Qdot605 (Invitrogen/Molecular Probes), FITC-conjugated CD169 (MOMA-1; AbD Serotech), biotinylated peanut agglutinin (Vector Laboratories), and biotinylated ED31 anti-MARCO.9 Images were collected with a Leica DM IRBE confocal laser scanning microscope (Leica Microsystems) equipped with 1 argon and 2 HeNe lasers, using an HC PL APO lens at 10×/0.40 CS and 20×/0.70 IMM CORR oil and 90% glycerol (MP Biomedicals). Images were processed with Adobe Photoshop CS4 Version 11.0.2 (Adobe Systems). The areas of GC (PNA+) and of follicular (B220+ cells surrounded by MOMA-1+ cells) areas were measured on images of random sections, and the ratio was calculated. Four mice per group were analyzed; the mean value of measurements from 2 images of each section was determined. Areas were quantified with the ImageJ software (National Institutes of Health).
Immunizations and (auto) Ab detection
Immunization with TNP–keyhole limpet hemocyanin (KLH) and analysis of total and high-affinity TNP-specific IgG responses in WT, B/WcKO, and WKO mice were performed as described.10
For immunization with a Streptococcus pneumoniae whole-cell vaccine (PnWCV), 100 μg of unencapsulated PnWCV was administered subcutaneously twice 2 weeks apart as described.11 Seven days after the boosting immunization, animals were bled, and serum IgG Abs specific for the vaccine were measured by ELISA as described.11
For immunization with Pneumovax23 (Merck), 1 μg of vaccine was administered in 200-μL volume intravenously. Serum was collected at the indicated days after immunization. To detect pneumococcal capsular polysaccharide-specific IgM and IgG Abs, ELISA plates were coated with a mixture of purified polysaccharides type 1, 2, 3, 4 and 7F (ATCC), each of them at the final coating concentration of 20 μg/mL. Detection of specific Abs was done as described for TNP-specific Abs.10
To measure phosphocholine-specific Abs, phosphocholine-ovalbumin was used to coat 96-well plates. Detection of phosphocholine-specific IgM in sera of naive mice was performed as described for TNP-specific Abs.10
To measure Ab response to ultraviolet (UV) light inactivated vesicular stomatitis virus (VSV), UV-inactivation of the VSV serotype Indiana was performed as described.12 PFU UV-inactivated VSV (2 × 108) was injected intravenously. Serum was obtained at the indicated times after immunization and was tested in a VSV neutralization assay as described.13
Ig serum concentrations were measured by ELISA according to the manufacturer's instruction (Zeptometrix) and as previously described.10
In vivo BrdU labeling
To detect in vivo–proliferating B lymphocytes, 2 mg of BrdU was injected intraperitoneally into Wasfl/+ mb1+/Cre heterozygous female mice (from now on referred to as hetB/WcKO mice). Twelve hours later, splenocytes were stained with the APC BrdU flow kit (BD Biosciences), according to the manufacturer's instructions.
Assessment of kidney glomerular damage
Kidneys from 6- to 17-month-old mice were fixed in 4% formalin for 24 hours, embedded in paraffin, and sectioned (3 μm) for periodic acid-Schiff staining performed with standard techniques. All slides were coded and evaluated in a blinded manner for identity of the sample. Tissues were visualized on a Nikon ECLIPSE 80i, and images were recorded with NIS-Elements BR 2.30 software (Nikon). Severity of the pathologic changes in the kidney was graded according to the presence of glomerular, interstitial, and vascular inflammation. Scores ranging from 0 (normal) to 3 (most severely inflamed) were assigned for each of the 3 features, as described.15,16 A minimum of 10 glomeruli were assessed in each mouse. The albumin/creatinine ratio was calculated by measuring separately urine creatinine by ELISA (R&D Systems) and albuminuria by ELISA (KAMIYA Biomedical Company). MRL/lpr mice served as positive controls for proteinuria.
Statistical analysis
Statistical significances were assessed by 1-way ANOVA or 2-way ANOVA with Bonferroni posttesting of significances between selected groups, Student t test or Mann-Whitney test as indicated in the figure legends.
Results
Generation of mice with B cell–specific deficiency of WASp
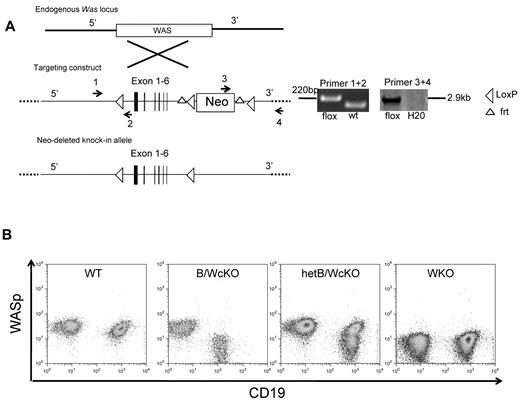
The strategy to generate Wasfl/fl mice is shown in Figure 1A. Briefly, a Was allele with exons 2-6 flanked by loxP sites and a neomycin-resistance cassette flanked by frt sites was introduced in embryonic stem cells by homologous recombination. The neo cassette was excised by breeding Was locus-targeted mice to B6.129S4-Gt(ROSA)26Sortm1(FLP1)Dym/RainJ transgenic mice expressing the FLP1-recombinase under the Rosa 26Sor promoter. This resulted in generation of Wasfl/fl mice that were bred to mb1-Cre knock-in mice8 to produce Wasfl/flmb1+/Cre female mice and Wasfl/Ymb1+/Cre male mice that lack expression of WASp in B cells but maintain normal expression in other lymphocyte subsets (Figure 1B) and myeloid cells (supplemental Figure 1A). Mice with B cell–specific deficiency of WASp are from now on referred to as B-cell intrinsic WASp conditional knock-out (B/WcKO) mice. Wasfl/+mb1+/Cre heterozygous female mice (hetB/WcKO mice) exhibited 2 populations of WASp+ and WASp− B cells because of X-chromosome inactivation (Figure 1B). WASp expression was lost at the pro-B cell (B220intCD43hi) stage in the BM of B/WcKO mice (supplemental Figure 1B). Enumeration of peripheral blood neutrophils, monocytes, and lymphocytes (including CD19+ B cells) was comparable in B/WcKO and in age-matched WT mice (supplemental Figure 1C). Interestingly, the significant thrombocytopenia observed in WKO mice was not detected in B/WcKO mice (supplemental Figure 1C).
Generation of a conditional mouse model with selective lack of WASp in B lymphocytes (B/WcKO mice). (A) Schematic representation of the targeting strategy. A Was allele with exons 2-6 flanked by loxP sites and a neomycin-resistance (Neo) cassette flanked by frt sites was introduced in embryonic stem cells by homologous recombination (targeting construct). Correct targeting was verified by PCR using appropriate primers. Was locus-targeted mice were bred to transgenic mice expressing the FLP1-recombinase under the Rosa 26Sor promoter, leading to excision of the Neo cassette and allowing generation of Wasfl/fl mice. (B) Representative flow cytometric analysis of expression of CD19 and WASp in peripheral blood lymphocytes of mice with the indicated genotype.
Generation of a conditional mouse model with selective lack of WASp in B lymphocytes (B/WcKO mice). (A) Schematic representation of the targeting strategy. A Was allele with exons 2-6 flanked by loxP sites and a neomycin-resistance (Neo) cassette flanked by frt sites was introduced in embryonic stem cells by homologous recombination (targeting construct). Correct targeting was verified by PCR using appropriate primers. Was locus-targeted mice were bred to transgenic mice expressing the FLP1-recombinase under the Rosa 26Sor promoter, leading to excision of the Neo cassette and allowing generation of Wasfl/fl mice. (B) Representative flow cytometric analysis of expression of CD19 and WASp in peripheral blood lymphocytes of mice with the indicated genotype.
B cell–specific deletion of WAS does not perturb B-cell development in the BM but affects distribution of peripheral B-cell subsets
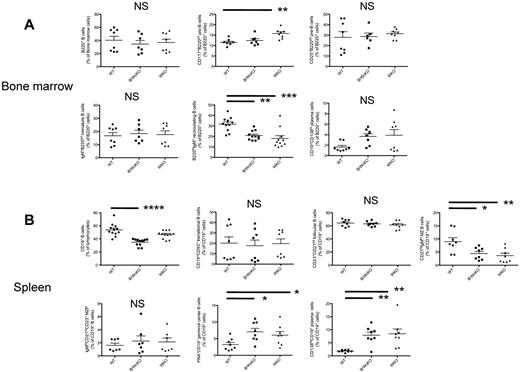
The relative distribution of various B-cell subpopulations in the BM and spleen from B/WcKO mice and age-matched WT and WKO mice was analyzed by flow cytometry (Figure 2; supplemental Figure 2). The BM of B/WcKO mice had a normal distribution of pro-B, pre-B, and immature B cells (Figure 2A). However, the percentage and absolute number of BM B220hi mature recirculating B cells were significantly reduced in both B/WcKO and WKO mice (20.9% ± 4.4% and 18.0% ± 9.1%) compared with WT mice (31.7% ± 6.8%; P < .01 and P < .001, respectively; Figure 2A; supplemental Figure 3A). In contrast, there was a nonsignificant trend to higher number and percentage of plasma cells (PCs) in the BM of B/WcKO and WKO mice compared with WT mice (Figure 2A; supplemental Figure 3A). In the spleen, both the proportion and the number of CD19+ B cells were significantly reduced in B/WcKO mice (Figure 2B; supplemental Figure 3B). Moreover, the percentage (among total CD19+ splenocytes) and the absolute number of MZ B cells were reduced 1.5- to 3-fold in B/WcKO and WKO mice compared with WT mice (Figure 2B; supplemental Figure 3B). MZ B cells derive from MZ precursor cells that retain expression of CD23. The number and relative proportion of MZ precursor B cells were similar in all mouse strains tested (Figure 2B; supplemental Figure 3B). The relative proportion of follicular B cells (among CD19+ splenocytes) was preserved in B/WcKO mice (Figure 2B), but their absolute number was reduced, consistent with overall B-cell lymphopenia (supplemental Figure 3B). In contrast, there was a 2- to 4-fold increase in the proportion of CD19+ PNA+ GC B cells (B/WcKO, 7.1% ± 2.9%; WKO, 6.1% ± 3.1%; WT, 3.2% ± 1.8%) and of CD19+CD138hi PCs (B/WcKO, 7.9% ± 3.4%; WKO, 8.5% ± 5.2%; WT, 1.8% ± 0.5%; Figure 2B). An increased percentage of GC B cells was also observed in lymph nodes of B/WcKO and WKO mice (supplemental Figure 4).
Proportion of B-lineage cell types in the BM and spleen. (A) Different B-cell subsets in the BM of WT, B/WcKO, and WKO mice were analyzed by flow cytometry with the indicated cell surface markers. Results from individual mice are shown. Statistical significance was assessed with 1-way ANOVA and Bonferroni posttest analysis. **P < .01 and ***P < .001. (B) Different B-cell subsets in the spleen of WT, B/WcKO, and WKO mice were analyzed by flow cytometry with the indicated cell surface markers. Data from individual mice are shown. Statistical significance was assessed with 1-way ANOVA and Bonferroni posttest analysis. *P < .05, **P < .01, and ****P < .0001.
Proportion of B-lineage cell types in the BM and spleen. (A) Different B-cell subsets in the BM of WT, B/WcKO, and WKO mice were analyzed by flow cytometry with the indicated cell surface markers. Results from individual mice are shown. Statistical significance was assessed with 1-way ANOVA and Bonferroni posttest analysis. **P < .01 and ***P < .001. (B) Different B-cell subsets in the spleen of WT, B/WcKO, and WKO mice were analyzed by flow cytometry with the indicated cell surface markers. Data from individual mice are shown. Statistical significance was assessed with 1-way ANOVA and Bonferroni posttest analysis. *P < .05, **P < .01, and ****P < .0001.
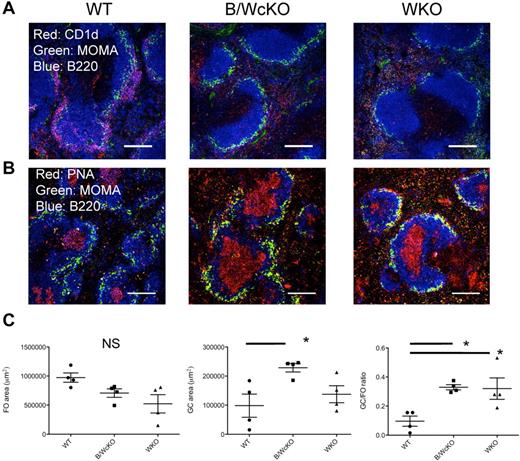
Abnormalities of the MZ and GC compartments in B/WcKO and WKO mice were confirmed by immunofluorescence stainings. The ring of B220+CD1d+ MZ B cells that normally surrounds MOMA-expressing metallophilic macrophages was almost absent in both WKO and B/WcKO mice (Figure 3A). To further confirm that WASp plays a cell-intrinsic role in MZ B-cell development, we analyzed the distribution of WASp+ and WASp− cells in B-cell splenocytes from hetB/WcKO mice. WASp− B cells accounted for 42% ± 11.2% of transitional B cells but for only 12.8% ± 6.9% of MZ B cells (P < .01; supplemental Figure 5), thus arguing for an in vivo advantage of WASp-expressing MZ B lymphocytes. Finally, consistent with the increased proportion of GC B cells detected by flow cytometry, GCs occupied a proportionally larger area of B-cell follicles in both naive B/WcKO and WKO mice (Figure 3B-C).
Reduction of MZ B cells and relative increase of GCs in the spleen of B/WcKO mice. (A) Splenic sections of WT, B/WcKO, and WKO mice were stained for B220 (blue), MOMA (green), and CD1d (red). MZ B cells (in purple) surround MOMA+ metallophilic macrophages in the spleen of WT mice but are severely depleted in B/WcKO and WKO mice. Scale bars, 150 μm. Eight mice per group were examined in 2 different experiments. Representative images are shown. (B) GC formation was assessed by staining for B220 (blue), PNA (red), and MOMA (green). Scale bars, 150 μm. Eight mice per group were examined in 2 different experiments. Representative images are shown. (C) The mean area occupied by follicles (FO; left panel) and by GCs (middle panel) is shown and was quantitated on immunohistologic slides as described in “Immunofluoresecence.” In addition, the GC/FO ratio of the respective areas is reported in the right panel. Values from individual spleen sections are shown. Significance was measured with 1-way ANOVA and Bonferroni posttest analysis. *P < .05.
Reduction of MZ B cells and relative increase of GCs in the spleen of B/WcKO mice. (A) Splenic sections of WT, B/WcKO, and WKO mice were stained for B220 (blue), MOMA (green), and CD1d (red). MZ B cells (in purple) surround MOMA+ metallophilic macrophages in the spleen of WT mice but are severely depleted in B/WcKO and WKO mice. Scale bars, 150 μm. Eight mice per group were examined in 2 different experiments. Representative images are shown. (B) GC formation was assessed by staining for B220 (blue), PNA (red), and MOMA (green). Scale bars, 150 μm. Eight mice per group were examined in 2 different experiments. Representative images are shown. (C) The mean area occupied by follicles (FO; left panel) and by GCs (middle panel) is shown and was quantitated on immunohistologic slides as described in “Immunofluoresecence.” In addition, the GC/FO ratio of the respective areas is reported in the right panel. Values from individual spleen sections are shown. Significance was measured with 1-way ANOVA and Bonferroni posttest analysis. *P < .05.
Dysgammaglobulinemia and impaired Ab response to T-independent Ags in B/WcKO mice
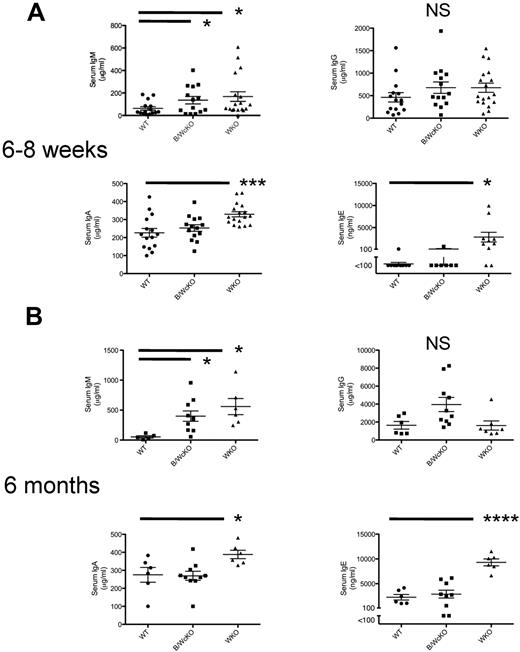
Analysis of serum Ig levels in 6- to 8-week-old showed that B/WcKO and WKO mice had normal levels of IgG but significantly increased serum IgM compared with WT mice (P < .05). WKO, but not B/WcKO, mice also showed increased serum levels of IgA and IgE (Figure 4A). These abnormalities were confirmed also in 6-month-old mice (Figure 4GB).
Analysis of dysgammaglobulinemia in B/WcKO mice. Serum from 6- to 8-week-old (A) or 6-month-old (B) naive WT, B/WcKO, and WKO mice was tested for total serum IgM, IgG, IgA, and IgE concentrations by ELISA. Values of individual mice are shown. Bars indicate mean ± SEM. Significance was assessed with the Student t test. *P < .05, ***P < .001, and ****P < .0001.
Analysis of dysgammaglobulinemia in B/WcKO mice. Serum from 6- to 8-week-old (A) or 6-month-old (B) naive WT, B/WcKO, and WKO mice was tested for total serum IgM, IgG, IgA, and IgE concentrations by ELISA. Values of individual mice are shown. Bars indicate mean ± SEM. Significance was assessed with the Student t test. *P < .05, ***P < .001, and ****P < .0001.
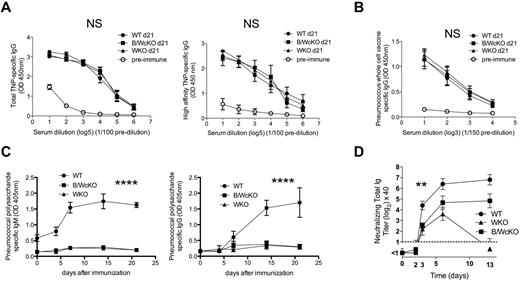
Both WKO and B/WcKO mice mounted a normal Ab response to the T cell–dependent Ag TNP-KLH (Figure 5A left panel), and the anti-TNP IgG Abs produced were of high affinity (Figure 5A right panel).
Normal follicular, but impaired MZ B-cell responses in B/WcKO mice. (A) WT, B/WcKO, or WKO mice were immunized intraperitoneally with 100 μg of TNP-KLH and challenged with 25 μg of TNP-KLH 14 days later. Seven days after TNP-challenge, TNP-specific IgG responses (total TNP-specific IgG response, left panel; high-affinity TNP-specific IgG response, right panel) were measured by ELISA. Preimmune serum from WT mice was used as a control. Results are reported as mean ± SEM of 5 mice per group. The differences were not significant with 2-way ANOVA and Bonferroni posttest analysis, P > .05. (B) WT, B/WcKO, and WKO mice were immunized subcutaneously with noncapsulated PnWCV twice 2 weeks apart. PnWCV-specific IgG responses were measured by ELISA in sera collected 1 week after the second immunization. Preimmune serum from WT mice was used as a control. Mean ± SEM values of 5 mice per group are shown. The differences were not significant with 2-way ANOVA and Bonferroni posttest analysis. P > .05. (C) WT, WKO, and B/WcKO mice were immunized intravenously with 1 μg of Pneumovax23 vaccine. At the indicated times, serum was tested by ELISA for pneumococcal capsular polysaccharide-specific IgM (left) and IgG (right) Abs. Mean ± SEM values of 5 mice per group are shown. Significance was assessed by 2-way ANOVA and Bonferroni posttest analysis. ****P < .0001. (D) WT, WKO, and B/WcKO mice were infected with 2 × 108 PFU UV-inactivated VSV. At the indicated times, serum was tested in a VSV neutralization assay for total neutralizing Ig. Mean ± SEM values of 5 mice per group are shown. Significances were assessed by 2-way ANOVA and Bonferroni posttest analysis. **P < .01.
Normal follicular, but impaired MZ B-cell responses in B/WcKO mice. (A) WT, B/WcKO, or WKO mice were immunized intraperitoneally with 100 μg of TNP-KLH and challenged with 25 μg of TNP-KLH 14 days later. Seven days after TNP-challenge, TNP-specific IgG responses (total TNP-specific IgG response, left panel; high-affinity TNP-specific IgG response, right panel) were measured by ELISA. Preimmune serum from WT mice was used as a control. Results are reported as mean ± SEM of 5 mice per group. The differences were not significant with 2-way ANOVA and Bonferroni posttest analysis, P > .05. (B) WT, B/WcKO, and WKO mice were immunized subcutaneously with noncapsulated PnWCV twice 2 weeks apart. PnWCV-specific IgG responses were measured by ELISA in sera collected 1 week after the second immunization. Preimmune serum from WT mice was used as a control. Mean ± SEM values of 5 mice per group are shown. The differences were not significant with 2-way ANOVA and Bonferroni posttest analysis. P > .05. (C) WT, WKO, and B/WcKO mice were immunized intravenously with 1 μg of Pneumovax23 vaccine. At the indicated times, serum was tested by ELISA for pneumococcal capsular polysaccharide-specific IgM (left) and IgG (right) Abs. Mean ± SEM values of 5 mice per group are shown. Significance was assessed by 2-way ANOVA and Bonferroni posttest analysis. ****P < .0001. (D) WT, WKO, and B/WcKO mice were infected with 2 × 108 PFU UV-inactivated VSV. At the indicated times, serum was tested in a VSV neutralization assay for total neutralizing Ig. Mean ± SEM values of 5 mice per group are shown. Significances were assessed by 2-way ANOVA and Bonferroni posttest analysis. **P < .01.
Similarly, B/WcKO mice vaccinated with PnWCV lacking the surface polysaccharide but otherwise carrying pneumococcal species cellular proteins11 developed comparable PnWCV-specific IgG responses compared with WKO and WT mice (Figure 5B).
In contrast, both IgM and IgG pneumococcus polysaccharide-specific Ab responses were virtually abrogated in B/WcKO and WKO mice vaccinated with a mixture of highly purified pneumococcal capsular polysaccharides (Pneumovax23) that are primarily recognized by marginal zone B cells17 (Figure 5C).
To further assess the consequences of MZ B-cell deficiency in B/WcKO mice, we immunized them with UV-inactivated VSV that is eliminated by a rapid, T-independent, MZ-dependent neutralizing IgM response.18,19 Abrogation of the early response (day 3 after immunization) was observed in both B/WcKO and WKO mice (P < .01 for both vs WT mice; Figure 5D). The higher neutralizing Ig response at later time points in B/WcKO mice compared with WKO mice probably reflects differences of WASp expression in, and function of, T helper cells in these 2 mouse models. Overall, these data indicate that lack of WASp expression in B cells interferes with the normal regulation of Ig production and impairs MZ-dependent Ab responses.
Elevated spontaneous secretion of “natural” IgM in B/WcKO mice
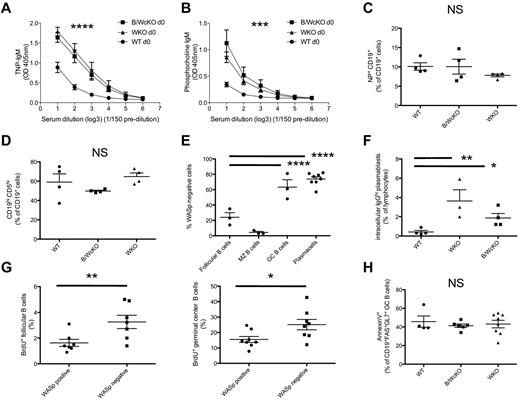
In addition to the elevated total serum IgM levels (Figure 4A-B), the serum of unimmunized (naive) B/WcKO and WKO mice contained increased amounts of IgM Abs specific for TNP (P < .0001; Figure 6A) and for phosphocholine (P < .001; Figure 6B). This spontaneous production of IgM Abs could reflect several, nonmutually exclusive, mechanisms, including (1) a higher frequency of TNP- and phosphocholine-specific B-cell splenocytes; (2) an increase in peritoneal B1 B cells20 ; and/or (3) skewing to terminal differentiation into PCs in WKO and B/WcKO mice (Figure 2B). The frequency of splenic B cells binding PE-conjugated NP hapten was comparable in WT, WKO, and B/WcKO mice (Figure 6C; supplemental Figure 6A). No expansion of peritoneal B1 cells (identified by expression of CD5 and/or CD11b) was observed in B/WcKO and WKO mice (Figure 6D; supplemental Figure 6B-C). To further investigate whether lack of WASp expression directly contributes to the expansion of GC B cells and PCs observed in B/WcKO mice, we analyzed the proportion of WASp+ and WASp− cells among follicular B cells, GC B lymphocytes, and PCs in the spleen of hetB/WcKO mice. In these mice, the majority of follicular B cells were WASp+ (only 24% ± 10.5% were WASp−), reflecting in vivo advantage of WASp+ peripheral mature B cells. However, in the same mice, 63% ± 16.6% of GC B cells and 73.8% ± 8.2% of PCs lacked WASp expression, implying a relative in vivo disadvantage for WASp-expressing cells within these 2 B-cell subsets (Figure 6E; supplemental Figure 6D). To determine whether WASp− B cells are more prone than WASp+ B cells to differentiate into PCs, splenocytes from WT, B/WcKO, and WKO mice were stimulated with the TLR9-ligand CpG, and plasmablast formation was analyzed 5 days later. In vitro stimulation of B/WcKO and WKO splenocytes with CpG resulted in generation of an increased proportion of class-switched plasmablasts compared with what was observed on stimulation of WT splenocytes (1.9% ± 0.9% and 3.6% ± 2.6% vs 0.4% ± 0.3%; P < .05; Figure 6F). To investigate the dynamics of GC expansion in B/WcKO mice, female hetB/WcKO mice were injected intraperitoneally with BrdU. After 12 hours the proportion of proliferating, BrdU+ cells within different B-cell subpopulations of each individual mouse was analyzed by flow cytometry. A higher proliferation rate was observed among WASp− compared with WASp+ follicular (3.3% ± 1.4% vs 1.6% ± 1.6%; P < .01) and GC (25.1% ± 9.5% vs 15.5% ± 5.5%; P < .05) B cells (Figure 6G). Finally, no difference in apoptosis among GC B cells from WT, B/WcKO, and WKO mice was observed by annexin V staining (Figure 6H). Taken together, these data indicate that lack of WASp expression in B cells facilitates proliferation of follicular B cells, expansion of the GC compartment, and generation of a higher fraction of Ab-secreting cells in vivo.
Increased spontaneous production of anti-TNP and anti-phosphocholine IgM Abs in B/WcKO mice. (A-B) Serum from naive WT, B/WcKO, and WKO mice was tested for IgM binding to TNP (A) and phosphocholine (B). Mean ± SEM values of 5 mice per group are shown. Significance was assessed by 2-way ANOVA and Bonferroni posttest analysis. ***P < .001 and ****P < .0001. (C) The proportion of NP-specific CD19+ splenic B cells in WT, WKO, and B/WcKO mice was analyzed by FACS by staining for cells reactive to PE-labeled NP hapten. Differences were not significant (NS) with 1-way ANOVA and Bonferroni posttest analysis. (D) The relative proportion of peritoneal CD19+ CD5+ B1 cells among peritoneal CD19+ B cells was assessed by flow cytometry in WT, WKO, and B/WcKO mice. The mean ± SEM for 4 mice per group is shown. Differences were not significant (NS) with 1-way ANOVA and Bonferroni posttest analysis. (E) Het-B/WcKO female mice were tested for the proportion of WASp+ versus WASp− cells within follicular B cell, MZ, GC, and PC B-cell compartments. The mean ± SEM values of 4-8 mice per group are shown. Statistical significance was assessed by 2-way ANOVA and Bonferroni posttest analysis. ****P < .0001. (F) Splenocytes of WT, B/WcKO, and WKO mice were stimulated in vitro with CpG (ODN 1826, 1.25μM final concentration). Five days later, generation of intracytoplasmic IgG+ (icIgGhi) plasmablasts was measured by flow cytometry. Bars indicate the mean ± SEM of icIgGhi plasmablasts generated in vitro from individual mice. Statistical significance was assessed by 1-way ANOVA and Bonferroni posttest analysis. *P < .05 and **P < .01. (G) HetB/WcKO mice were injected intraperitoneally with 2 mg of BrdU. Twelve hours later, BrdU incorporation in WASp+ versus WASp− fractions of follicular B cells (left) and GC B cells (right) was measured by BrdU-specific Ab. Data of individual mice are shown. Statistical significance was assessed by Student t test. *P < .05 and **P < .01. (H) Apoptosis of GC B cells was assessed by annexin V labeling gating on CD19+ FAS+ GL7+ GC B cells from WT, B/WcKO, and WKO mice.
Increased spontaneous production of anti-TNP and anti-phosphocholine IgM Abs in B/WcKO mice. (A-B) Serum from naive WT, B/WcKO, and WKO mice was tested for IgM binding to TNP (A) and phosphocholine (B). Mean ± SEM values of 5 mice per group are shown. Significance was assessed by 2-way ANOVA and Bonferroni posttest analysis. ***P < .001 and ****P < .0001. (C) The proportion of NP-specific CD19+ splenic B cells in WT, WKO, and B/WcKO mice was analyzed by FACS by staining for cells reactive to PE-labeled NP hapten. Differences were not significant (NS) with 1-way ANOVA and Bonferroni posttest analysis. (D) The relative proportion of peritoneal CD19+ CD5+ B1 cells among peritoneal CD19+ B cells was assessed by flow cytometry in WT, WKO, and B/WcKO mice. The mean ± SEM for 4 mice per group is shown. Differences were not significant (NS) with 1-way ANOVA and Bonferroni posttest analysis. (E) Het-B/WcKO female mice were tested for the proportion of WASp+ versus WASp− cells within follicular B cell, MZ, GC, and PC B-cell compartments. The mean ± SEM values of 4-8 mice per group are shown. Statistical significance was assessed by 2-way ANOVA and Bonferroni posttest analysis. ****P < .0001. (F) Splenocytes of WT, B/WcKO, and WKO mice were stimulated in vitro with CpG (ODN 1826, 1.25μM final concentration). Five days later, generation of intracytoplasmic IgG+ (icIgGhi) plasmablasts was measured by flow cytometry. Bars indicate the mean ± SEM of icIgGhi plasmablasts generated in vitro from individual mice. Statistical significance was assessed by 1-way ANOVA and Bonferroni posttest analysis. *P < .05 and **P < .01. (G) HetB/WcKO mice were injected intraperitoneally with 2 mg of BrdU. Twelve hours later, BrdU incorporation in WASp+ versus WASp− fractions of follicular B cells (left) and GC B cells (right) was measured by BrdU-specific Ab. Data of individual mice are shown. Statistical significance was assessed by Student t test. *P < .05 and **P < .01. (H) Apoptosis of GC B cells was assessed by annexin V labeling gating on CD19+ FAS+ GL7+ GC B cells from WT, B/WcKO, and WKO mice.
Elevated autoantibody production in B/WcKO mice
Increased occurrence of autoimmune manifestations and autoantibody production has been reported both in patients with WAS,21 and in WKO mice.22 However, it remains unclear whether this reflects, at least in part, B cell–intrinsic immune dysregulation, or whether it is because of B cell–extrinsic mechanisms, such as impaired function of regulatory T cells,23 defective function of dendritic cells,24 and /or abnormalities of apoptosis.22
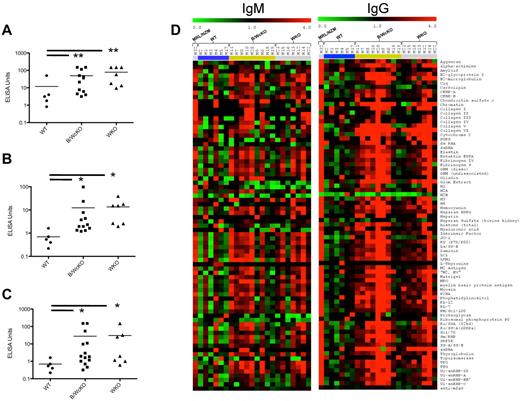
When assessed by ELISA, markedly elevated levels of IgG Abs to ssDNA, dsDNA, and chromatin were detected in B/WcKO and in WKO mice (Figure 7A-C). To analyze the diversity of autoantibody production in WASp-deficient mice, sera from 6-month-old WT, B/WcKO, and WKO mice were screened for 73 distinct autoantibody specificities with the use of an autoantibody array. IgM and IgG autoantibodies to a broad range of self-Ags were detected in both B/WcKO and WKO mice (Figure 7D). To examine the pathologic relevance of increased autoantibody production, glomerular damage was assessed by a “blinded” nephropathologist in sections from 11 WT, 11 B/WcKO, and 14 WKO mice (of 6-17 months of age) with the use of an established scoring system.15,16 A variable degree of mesangial cell proliferation and mesangial matrix expansion was observed in 4 of 11 B/WcKO mice, 9 of 14 WKO mice, and 1 of 11 WT mice and was more commonly seen in mice older than 10 months (supplemental Figure 7A-B). Although the glomerular damage score was higher in B/WcKO and WKO mice than in WT mice (P < .05 for both), in most cases the damage was modest. Only 1 of 11 B/WcKO mice and 1 of 14 WKO mice presented wire-loop capillary lesions. Furthermore, the albumin/creatinine ratio, indicative of impaired glomerular function, was not significantly different in B/WcKO, WKO, and WT mice, although there was a tendency for older B/WcKO mice to develop proteinuria (supplemental Figure 7C). Finally, no cases of death because of nephritis were observed in B/WcKO and WKO mice followed for ≤ 17 months.
Increased autoantibody production in B/WcKO mice. (A-C) Sera of 6-month-old WT, B/WcKO, and WKO mice were tested by ELISA for IgG Abs specific for anti-ssDNA (A), anti-dsDNA (B), and chromatin (C). Data of individual mice are shown. Significance was calculated with the Mann-Whitney test. *P < .05 and **P < .01. (D) An autoantibody array chip was used to detect autoantibodies of IgM (left) or IgG (right) isotype against 73 different autoantigens (listed on the right). Sera from 6 WT mice (blue bar), 9 B/WcKO mice (green bar), and 7 WKO mice (gray bar) were tested. A serum from a lupus-prone MRL/NZM mouse (first row) served as a positive control. A red dot in the array indicates a 4-fold increase of autoantibody titer compared with average values in control sera.
Increased autoantibody production in B/WcKO mice. (A-C) Sera of 6-month-old WT, B/WcKO, and WKO mice were tested by ELISA for IgG Abs specific for anti-ssDNA (A), anti-dsDNA (B), and chromatin (C). Data of individual mice are shown. Significance was calculated with the Mann-Whitney test. *P < .05 and **P < .01. (D) An autoantibody array chip was used to detect autoantibodies of IgM (left) or IgG (right) isotype against 73 different autoantigens (listed on the right). Sera from 6 WT mice (blue bar), 9 B/WcKO mice (green bar), and 7 WKO mice (gray bar) were tested. A serum from a lupus-prone MRL/NZM mouse (first row) served as a positive control. A red dot in the array indicates a 4-fold increase of autoantibody titer compared with average values in control sera.
Discussion
Immunodeficiency, thrombocytopenia, allergic manifestations, including high serum IgE and autoimmunity, are hallmarks of WAS.1,25,26 Most of these features are recapitulated in WKO mice.3,6,27 Loss of WASp expression impairs the function of all immune cell lineages tested to date, making it difficult to clarify the relative contribution of individual cell types to WAS-associated immunodeficiency and immune dysregulation in vivo. To address this, we generated a novel mouse model that used the Cre-Lox technology for lineage-specific deletion of WASp expression. Cross-breeding with an mb1-Cre strain8 successfully and specifically deleted WASp expression in B-lineage lymphocytes from an early stage of development, permitting us to study the effects of B cell–restricted deficiency of WASp on immune function. Abnormalities of humoral immunity found in patients with WAS include dysgammaglobulinemia and defects of specific Ab production,28,29 in particular in response to T-independent polysaccharide Ags.30 Previous studies had shown that WASp is required for several functions in B cells, such as cytoskeletal reorganization, adhesion, migration, and BCR-mediated,4,5,31 although the physiologic importance of these findings for in vivo immune responses has not been investigated.
Analysis of B-cell development and Ab production in the BM and secondary lymphoid tissues of patients with WAS is challenging. Analysis of WKO mice has found significant defects of Ab responses to T-independent Ags, similar to that observed in humans.5,6 Furthermore, a nonredundant role for WASp has been found in B-cell homeostasis, particularly at later stages of B-cell differentiation.2,6 Although WASp is not required for development and survival of early pre- or pro-B cells, significant survival advantage has been observed in WASp+ mature recirculating B cells and MZ B cells.2,5,6,32,33
With the use of a B cell–specific model of WASp deficiency, we have confirmed that the defects of B-cell homeostasis previously described in WKO mice result from B-cell intrinsic mechanisms and not from inadequate survival and/or proliferation signals delivered by other cell types. B/WcKO mice, like WKO mice, exhibit reduced recirculating B cells in the BM, as well as a reduced number of splenic MZ B cells. MZ B and recirculating B cells express higher relative levels of WASp than other B-cell subsets, which is consistent with a greater functional requirement for WASp in these cell types.2 It has been previously reported, and confirmed here, that WKO mice have a normal2 or slightly reduced6 number of follicular B cells. Unexpectedly, the absolute number of follicular B cells was significantly reduced in the spleen of B/WcKO mice because of a striking relative reduction of B cells among total splenocytes. Along with data showing enrichment for WASp-expressing follicular B cells in hetB/WcKO mice, these data suggest that lack of WASp expression in the B-cell lineage confers a disadvantage during follicular B-cell development. This reduction in the number of follicular B cells may contribute to the lower number of recirculating mature B cells detected in the BM of B/WcKO mice. However, a reduced number of BM recirculating mature B cells was observed also in WKO mice that have a normal number of follicular mature B cells. It is possible that defective migration of WASp-deficient B cells in response to chemokines and other chemoattractors may also contribute to the reduced number of mature B cells in the BM.
Detailed examination of the splenic architecture in B/WcKO mice confirmed a significant reduction in MZ B cells, even though the number and arrangement of MZ macrophages were normal. This finding showed that defects of MZ organization and function in WASp-deficient mice are primarily because of B cell–intrinsic abnormalities. Several mechanisms may contribute to poor retention and/or positioning of MZ B cells in vivo, including impaired migration of B lymphocytes in response to S1P and CXCL13,5,6,34 defective adhesive response to ICAM-1 and VCAM-1, and impaired formation of a mature immunologic synapse in response to BCR signaling in the presence of ICAM-1.2,5,35-37
Abnormalities of Ig serum levels have been reported in patients with WAS, which most often include elevated IgA and IgE levels.28,29 In this study, we demonstrated significantly elevated levels of IgM, IgA, and IgE (the latter not previously reported) in WKO mice, whereas only IgM levels were high in B/WcKO mice. This implies that abnormalities of non-B cells, probably dendritic cells and/or regulatory T cells,38 might be responsible for elevated IgA and IgE in WKO mice, whereas B cell–intrinsic dysregulation accounts for increased serum IgM in vivo. Consistent with previous observations in WKO mice,3 B/WcKO mice mounted a normal Ab response to T-dependent Ags but reduced responses to prototypic type II T-independent Ags. These findings show that expression of WASp in B lymphocytes is required for adequate Ab response to T-independent Ags and suggest that reconstitution of WASp expression in B lymphocytes after hematopoietic cell transplantation or gene therapy is needed to completely prevent the risk of overwhelming infections because of encapsulated pathogens.
Impaired B-cell tolerance, with production of autoantibodies, is a feature of both human and murine WASp deficiency21,22 and may contribute to development of autoimmune complications. Autoimmunity has been reported in 24%-72% of patients with WAS in various series, with autoimmune cytopenias, vasculitis, renal disease, inflammatory bowel disease, and arthritis being the most common manifestations.21,29,39,40 We have previously reported that WKO mice produce elevated levels of IgM specific for phosphocholine and for the hapten TNP.6 We have now observed similar findings in B/WcKO mice, indicating that dysregulated production of spontaneous IgM Abs is because of a B cell–intrinsic defect. We have shown that this was neither because of a higher frequency of TNP- and phosphocholine-specific B-cell splenocytes or to an increase in natural IgM-secreting peritoneal B1 cells but was related to an expansion of GCs and PCs seen in both B/WcKO and WKO mice. In hetB/WcKO mice, in which WASp-expressing B cells compete with their WASp-negative counterparts, we observed a striking overrepresentation of WASp-deficient B cells in both the PC and GC compartments. With the use of in vivo BrdU-labeling experiments in hetB/WcKO mice, we have shown that WASp− follicular and GC B cells have higher proliferation activity than their WASp+ counterpart. Furthermore, a higher number of terminally differentiated, class-switched plasmablasts were generated after in vitro CpG stimulation of WASp− B cells. Altogether, these findings support the hypothesis that WASp normally functions to negatively regulate the spontaneous formation of GCs and differentiation into Ab-producing cells. Increased production of antinuclear, anti-ssDNA, and anti-dsDNA IgM and IgG Abs, as well as of tissue-specific IgG autoantibodies, which may associate with tissue damage (glomerulonephritis), had been reported in WKO mice.22,32 We have observed that production of autoantibodies with a broad range of specificity (including both tissue-specific and ubiquitous Ags) is a prominent feature of both B/WcKO and WKO mice. Although a possible role for B-cell extrinsic factors, such as impaired T-regulatory or dendritic cell function,23,24,41-45 in the immune dysregulation and autoantibody production of WAS cannot be ruled out, our data show that WASp deficiency in B cells is sufficient to promote autoantibody production. A recent report came to the same conclusion while analyzing mixed chimeric mice with the use of WKO and B-cell deficient μMT-mouse BM transfer into irradiated recipients.7 In that model, a large fraction of recipient mice with WASp-negative B lymphocytes developed glomerulonephritis and died prematurely.7 Similarly, Nikolov et al have reported development of proliferative glomerulonephritis with immune complex deposition in aged WKO mice.22 In contrast, Bosticardo et al have recently reported that production of autoantibodies is not generally associated with tissue damage in WKO mice.32 In our study, we have observed mesangial proliferation in a significant fraction of B/WcKO and WKO mice versus WT mice. Kidney disorder was more evident in older B/WcKO and WKO mice, especially if older than 10 months. However, kidney involvement was generally modest, and in most cases it was not associated with significant proteinuria. In contrast, significantly increased albumin/creatinine ratio was reported by Becker-Herman et al in a chimeric mouse model of WASp deficiency in B cells7 and by Nikolov et al in aged WKO mice.32 Several reasons may account for these discrepancies. In the chimeric mouse model reported by Becker-Herman et al,7 irradiation-induced apoptosis and lymphopenia-associated homeostatic proliferation may both contribute to induction of autoimmunity and tissue damage.46,47 Furthermore, the autoimmune-prone WKO mice reported by Nikolov et al22 were on a Sv129 X C57BL/6 mixed background,22 which is known to be more predisposed to immune dysregulation.48 Nonetheless, our data indicate that selective lack of WASp expression in B lymphocytes predisposes to phenotypically manifest autoimmune disease.
In conclusion, we have shown that intrinsic defects of B-cell function are responsible and sufficient for many of the abnormalities of humoral immunity described in WASp deficiency, including defects of Ab production to T-independent Ags and a loss of B-cell tolerance. These findings define an important role for B lymphocytes in the pathophysiology of WAS, and they indicate that reconstitution of WASp expression in B lymphocytes should be pursued after hematopoietic cell transplantation or gene therapy for WAS. Furthermore, defective cytoskeletal function in the B-cell lineage may contribute to other autoimmune conditions, including those for which B-cell depletion strategies have been shown to be therapeutically useful.
Finally, through breeding with relevant Cre-expressing mice, the conditional mouse model described here may enable careful dissection of the cell type–specific role played by WASp in immune function and homeostasis in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grant 2PO1HL059561-11-A1, L.D.N.) and the Manton Foundation (L.D.N.); the Swiss National Science Foundation (SNSF/SSMBS; grant PASMP3-127678, M.R.); the Karolinska Institutet (L.W.); the Swedish Research Foundation (L.W.); FIS-ISCIII (grant PI10/02 511, M.A.d.l.F.); Consejería de Educación (M.A.d.l.F.; grant VA244A11-2, J.d.C.y.L.); the Wellcome Trust (A.J.T. and G.B.); and by a grant from the Primary Immunodeficiency Association through the Academy of Medical Sciences (S.O.B.).
National Institutes of Health
Authorship
Contribution: M.R. performed and designed most experiments and contributed to the writing of the paper; S.O.B., M.A.d.l.F., G.B., and S.V. performed research and contributed to the writing; C.D., J.E.W., D.M., N.H., P.A.L., L.P., H.F., M.M., E.C. O.M.D., F.F., A.B.F.F., E.M.L., M.J.M., K.S.L., and L.W. performed experiments; K.M., M.K., F.C., K.N., D.B., and R.M. contributed vital experimental tools; G.C.T., J.H., J.M., C.T., R.S.G., S.S., and L.W. designed and supervised some experiments; and A.J.T. and L.D.N. designed, analyzed, and supervised all experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adrian J. Thrasher, Molecular Immunology Unit, Centre for Immunodeficiency, UCL Institute of Child Health, 30 Guilford St, London, WC1N 1EH, United Kingdom; e-mail: a.thrasher@ich.ucl.ac.uk; or Luigi D. Notarangelo, Division of Immunology, Children's Hospital Boston, Karp Research Bldg, 1 Blackfan Cir, Boston, MA 02115; e-mail: luigi.notarangelo@childrens.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal