Abstract
Community respiratory viruses are significant causes of morbidity and mortality in patients with leukemia and hematopoietic stem cell transplant (HSCT) recipients. Data on characteristics and outcomes of parainfluenza virus (PIV) infections in these patients are limited. We reviewed the records of patients with leukemia and HSCT recipients who developed PIV infections to determine the characteristics and outcomes of such infections. We identified 200 patients with PIV infections, including 80 (40%) patients with leukemia and 120 (60%) recipients of HSCT. At presentation, most patients (70%) had an upper respiratory tract infection and the remaining patients (30%) had pneumonia. Neutropenia, APACHE II score more than 15, and respiratory coinfections were independent predictors of progression to pneumonia on multivariate analysis. Overall mortality rate was 9% at 30 days after diagnosis and 17% among patients who had PIV pneumonia, with no significant difference between patients with leukemia and HSCT recipients (16% vs 17%). On multivariate analysis, independent predictors of death were relapsed or refractory underlying malignancy, APACHE II score more than 15, and high-dose steroid use. Patients with leukemia and HSCT are at risk for serious PIV infections, including PIV pneumonia, with a significant mortality rate. We identified multiple risk factors for progression to pneumonia and death.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2969.
Disclosures
The authors, the Associate Editor Martin S. Tallman, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe the prevalence and clinical characteristics of parainfluenza virus (PIV) infections in patients with leukemia or HSCT, based on a medical record review.
Describe outcomes of PIV infections in patients with leukemia or HSCT and the factors predicting progression to pneumonia, based on a medical record review.
Describe risk factors for mortality from PIV infections in patients with leukemia or HSCT, based on a medical record review.
Release date: March 22, 2012; Expiration date: March 22, 2013
Introduction
For many years, infections with community-acquired respiratory viruses, such as influenza, parainfluenza virus (PIV), respiratory syncytial virus, and picornavirus, were not recognized as a possible cause of serious infections in patients with cancer. However, during the past 2 decades and with the availability of better microbiologic diagnostic tools (ie, molecular testing), multiple studies reported on these viruses causing significant morbidity and mortality, especially in patients with hematologic malignancies and those undergoing hematopoietic stem cell transplantation (HSCT).1-9
PIV is a common cause of viral infection in patients with hematologic malignancies, mainly leukemia, and recipients of HSCT.4-6,10 In these patients, PIV infection may exhibit several distinct characteristics not typically seen in the general population, such as prolonged duration of infection or shedding, nonspecific initial presentation, and progressive respiratory failure.1,5,6,11,12 This virus also may spread to the lower respiratory tract, with viral pneumonia developing in up to 50% of patients1,4,10,13,14 and having a reported mortality rate of up to 46%.1,4,5,14,15 Multiple risk factors for progression to pneumonia and/or associated mortality were identified in this patient population, including severe lymphopenia, concomitant other respiratory infections, PIV type III, and receipt of corticosteroids at the time of PIV infection acquisition.4,5,14
There is currently no licensed antiviral drug for the treatment of PIV infections. Ribavirin is active against PIV in vitro but possesses only marginal activity in vivo. Reports on the efficacy of aerosolized, intravenous, or oral ribavirin in patients with PIV pneumonia have been mixed.1,4,5,10,15-17
Reports comparing the impact of PIV infection in patients with leukemia and HSCT recipients are lacking. We performed a retrospective study to compare the clinical course of PIV and risk factors for progression to pneumonia and/or death, with or without therapy, after PIV infection in these 2 patient populations.
Methods
Patients and data collected
From the infection control database at our institution, we identified all consecutive adult patients with PIV infection who had underlying leukemia and/or were recipients of HSCT who had received care at our institution from October 2002 to November 2007. At our institution, symptomatic patients are screened for respiratory viruses by obtaining the products of nasal washes and/or bronchoalveolar lavage and submitting them for direct immunofluorescence antigen detection for respiratory syncytial virus and influenza A virus and using shell vial cultures technique to detect PIV and other respiratory viruses as well. On admission, chest radiograph or CT scan of chest is done on all patients with or suspected of having respiratory viral infections, or at any time when pneumonia is suspected. All hospitalized patients with upper respiratory symptoms are placed on respiratory isolation. The study was approved by The University of Texas MD Anderson Cancer Center institutional review board.
Data collected included demographic information (age, sex, and race), underlying malignancy, date of diagnosis, clinical staging, HSCT status, past medical history, comorbidities, date of diagnosis of PIV infection, hospital admission date (if applicable), length of hospital stay, and APACHE II score at the onset of infection. Laboratory values that were collected included absolute neutrophil count (ANC) and absolute lymphocyte count (ALC) within a week of diagnosis of PIV infection. Radiographic data, including CT images of the chest and chest x-ray findings, were collected, as well as pertinent microbiologic data. We also collected data regarding treatment with immunosuppressive and cytotoxic therapy, including chemotherapy, radiation therapy, and cumulative dose of steroids, within 4 weeks of diagnosis of PIV infection.
The use of antiviral therapy to treat the infection was recorded, including the drug, route of administration, start date, duration, and dosage, along with concurrent antimicrobial therapy and adjunctive treatment (eg, the use of intravenous immunoglobulins [IVIGs]). Information about any adverse drug reactions resulting from antiviral therapy was also collected. Finally, outcomes were recorded as complete resolution of infection, progressive infection, or death (see “Definitions”). Each patient underwent follow-up for at least 30 days from the date of diagnosis of PIV infection. For allogeneic transplantation, GVHD prophylaxis consisted of tacrolimus (Fujisawa Healthcare) started on day −2 and administered for 6 to 9 months if no GVHD, and mini-methotrexate (5 mg/m2 on posttransplantation days 1, 3, 6, and 11).
Definitions
Upper respiratory tract infection (URI) was defined as the presence of symptoms, such as coryza, rhinorrhea, pharyngitis, cough, otitis media, and/or sinusitis in association with parainfluenza culture from nasal washings, with no evidence of new and/or progressive infiltrates on chest diagnostic imaging. Lower respiratory tract infection (LRI) or pneumonia was defined as the presence of signs and symptoms that included cough, dyspnea, and hypoxia in association with new interstitial infiltrates on chest diagnostic imaging and the isolation of PIV from bronchoalveolar lavage fluid and/or nasal washings. Cases were classified as community-acquired if symptoms developed before hospitalization or within the first 2 days after admission or as nosocomial if symptoms developed any time after that during hospitalization. Neutropenia was defined as an ANC less than 500 cells/μL of blood. Lymphopenia was defined as an ALC less than 200 cells/μL of blood.
Complete resolution of PIV infection was defined as the complete resolution of signs and symptoms of PIV infection, with or without complete radiologic improvement.
Progressive infection was defined as worsening of signs or symptoms and/or progression to pneumonia or death associated with PIV infection within 30 days of diagnosis.
Statistical analysis
The χ2 or Fisher exact test was used for categorical data analysis. Continuous variables were compared using Wilcoxon rank-sum tests. Survival analysis was performed on progression to pneumonia. The patients were followed up for 30 days after URI presentation. The probability of being free from progressing to pneumonia was estimated for the patients with leukemia and those with HSCT using Kaplan-Meier approach, and was compared between the 2 groups using a log-rank test. A similar analysis of patient mortality within 30 days after diagnosis of PIV infection was also performed. In addition, the multiple logistic regression method was used to evaluate the independent effects of factors, such as antiviral treatment and underlying condition (leukemia or HSCT) on the progression to pneumonia and mortality, as necessary (see Tables 2 and 5). First, univariate analyses were performed to evaluate the predictive effect of each factor alone. Then, any factor with a P value less than .25 was included in a full multiple logistic regression model. Finally, the full model was reduced by a backward elimination procedure at a 5% significance level. The only exception was the factor “steroid usage of more than or equal to 600 mg prednisolone or equivalent” in the multiple logistic regression analysis of patient mortality within 30 days of diagnosis. We noticed that this factor was statistically significant in the logistic regression analysis of patient mortality within 60 days of diagnosis (data not shown). Therefore, although in the univariate analysis of mortality within 30 days of diagnosis it had a P value more than .25, we still included it in the full multivariate model, and it proved to be a significant factor in the final logistic regression model. All tests were 2-sided, and the statistical significance was set at P less than or equal to .05. All statistical analyses were performed using SAS Version 9.1 (SAS Institute).
Results
Patients and baseline/disease characteristics
We identified 200 patients with PIV infections at an incidence of 1% (80 of 7745) for patients with leukemia, 1.3% for autologous HSCT recipients (23 of 1717), and 5.5% for allogeneic HSCT recipients (97 of 1756) during the study period. There was almost equal representation of males and females (52% vs 48%, respectively). The median age was 55 years (range, 17-84 years), and most of the patients were white (153, 77%). Eighty patients (40%) had leukemia and 120 (60%) patients had previous HSCT at the time of presentation with PIV infection (Table 1). Acute myeloid leukemia (44%) and acute lymphoblastic leukemia (28%) were the most common malignancies among patients with leukemia. Among those who underwent HSCT, 81% received allogeneic and 19% had autologous transplants. The median time from HSCT to diagnosis of PIV infection was 70 days (range, 0-2836 days). At diagnosis, the median APACHE II score for patients with leukemia was 14, compared with 10 for HSCT recipients (P < .0001).
Characteristics of patients with leukemia and HSCT recipients with PIV infection
| Characteristic . | Leukemia* (n = 80), N (%) . | HSCT recipients* (n = 120), N (%) . | P . |
|---|---|---|---|
| Sex, male | 39 (49) | 66 (55) | .39 |
| Median age, y (range) | 57 (17-84) | 54 (20-82) | .28 |
| Underlying malignancy | |||
| Acute myeloid leukemia | 35 (44) | 41 (34) | |
| Acute lymphocytic leukemia | 22 (28) | 3 (3) | |
| Chronic myeloid leukemia | 7 (9) | 7 (6) | |
| Chronic lymphoid leukemia | 12 (15) | 13 (11) | |
| Non-Hodgkin lymphoma | — | 21 (18) | |
| Hodgkin lymphoma | — | 6 (5) | |
| T-cell prolymphocytic leukemia | 2 (3) | 0 (0) | |
| MDS | 2 (3) | 2 (2) | |
| Multiple myeloma | — | 21 (18) | |
| Solid tumors | — | 6 (5) | |
| HSCT recipients | |||
| Autologous | — | 23 (19) | |
| Allogeneic | — | 97 (81) | |
| Matched related donors | — | 37/97 (38) | |
| Mismatched related donors | — | 3/97 (3) | |
| Unrelated donors | — | 57/97 (59) | |
| Median time from transplantation to PIV infection, d (range) | — | 70 (0-2836) | |
| Presence of GVHD at presentation | — | 45 (38) | |
| Active GVHD | — | 27/45 (60) | |
| Chronic GVHD | — | 18/45 (40) | |
| Site of infection at presentation | .16 | ||
| URI | 52 (65) | 89 (74) | |
| Pneumonia | 28 (35) | 31 (26) | |
| Acquisition of infection | .75 | ||
| Community acquired | 68 (85) | 100 (83) | |
| Nosocomially acquired | 12 (15) | 20 (17) | |
| Type of PIV | .32 | ||
| Type I | 12 (15) | 10 (8) | |
| Type II | 2 (3) | 4 (3) | |
| Type III | 66 (82) | 106 (88) | |
| Neutropenia at the time of diagnosis of PIV (ANC < 500/μL) | 38/80 (48) | 13/115 (11) | < .0001 |
| Lymphopenia at the time of diagnosis of PIV (ALC < 200/μL) | 37/80 (46) | 29/114 (25) | .003 |
| Receipt of corticosteroids within a month of diagnosis of PIV | 28 (35) | 94 (78) | < .0001 |
| Receipt of chemotherapy within a month of diagnosis of PIV | 77 (96) | 35 (29) | < .0001 |
| APACHE II score at the time of diagnosis of PIV, median (range) | 14 (5-26) | 10 (3-30) | < .0001 |
| Progression from URI to pneumonia | 49/80 (61) | 46/119 (39) | .002 |
| Hospitalization resulting from PIV infection | 58/75 (77) | 40/110 (36) | < .0001 |
| Length of hospital stay, median (range) | 10 (1-39) | 9 (1-53) | .86 |
| Respiratory coinfections within a month of diagnosis of PIV infection† | 21 (26) | 35 (29) | .78 |
| ICU stay | 10 (13) | 7 (6) | .1 |
| Mechanical ventilation | 8 (10) | 5 (4) | .1 |
| Resolution of PIV infection | 68/79 (86) | 96/108 (89) | .56 |
| Days from diagnosis of PIV infection to response, median (range) | 8 (1-92) | 13 (1-64) | .001 |
| Mortality within 30 days of PIV infection | 8/79 (10) | 8/108 (7) | .7 |
| Characteristic . | Leukemia* (n = 80), N (%) . | HSCT recipients* (n = 120), N (%) . | P . |
|---|---|---|---|
| Sex, male | 39 (49) | 66 (55) | .39 |
| Median age, y (range) | 57 (17-84) | 54 (20-82) | .28 |
| Underlying malignancy | |||
| Acute myeloid leukemia | 35 (44) | 41 (34) | |
| Acute lymphocytic leukemia | 22 (28) | 3 (3) | |
| Chronic myeloid leukemia | 7 (9) | 7 (6) | |
| Chronic lymphoid leukemia | 12 (15) | 13 (11) | |
| Non-Hodgkin lymphoma | — | 21 (18) | |
| Hodgkin lymphoma | — | 6 (5) | |
| T-cell prolymphocytic leukemia | 2 (3) | 0 (0) | |
| MDS | 2 (3) | 2 (2) | |
| Multiple myeloma | — | 21 (18) | |
| Solid tumors | — | 6 (5) | |
| HSCT recipients | |||
| Autologous | — | 23 (19) | |
| Allogeneic | — | 97 (81) | |
| Matched related donors | — | 37/97 (38) | |
| Mismatched related donors | — | 3/97 (3) | |
| Unrelated donors | — | 57/97 (59) | |
| Median time from transplantation to PIV infection, d (range) | — | 70 (0-2836) | |
| Presence of GVHD at presentation | — | 45 (38) | |
| Active GVHD | — | 27/45 (60) | |
| Chronic GVHD | — | 18/45 (40) | |
| Site of infection at presentation | .16 | ||
| URI | 52 (65) | 89 (74) | |
| Pneumonia | 28 (35) | 31 (26) | |
| Acquisition of infection | .75 | ||
| Community acquired | 68 (85) | 100 (83) | |
| Nosocomially acquired | 12 (15) | 20 (17) | |
| Type of PIV | .32 | ||
| Type I | 12 (15) | 10 (8) | |
| Type II | 2 (3) | 4 (3) | |
| Type III | 66 (82) | 106 (88) | |
| Neutropenia at the time of diagnosis of PIV (ANC < 500/μL) | 38/80 (48) | 13/115 (11) | < .0001 |
| Lymphopenia at the time of diagnosis of PIV (ALC < 200/μL) | 37/80 (46) | 29/114 (25) | .003 |
| Receipt of corticosteroids within a month of diagnosis of PIV | 28 (35) | 94 (78) | < .0001 |
| Receipt of chemotherapy within a month of diagnosis of PIV | 77 (96) | 35 (29) | < .0001 |
| APACHE II score at the time of diagnosis of PIV, median (range) | 14 (5-26) | 10 (3-30) | < .0001 |
| Progression from URI to pneumonia | 49/80 (61) | 46/119 (39) | .002 |
| Hospitalization resulting from PIV infection | 58/75 (77) | 40/110 (36) | < .0001 |
| Length of hospital stay, median (range) | 10 (1-39) | 9 (1-53) | .86 |
| Respiratory coinfections within a month of diagnosis of PIV infection† | 21 (26) | 35 (29) | .78 |
| ICU stay | 10 (13) | 7 (6) | .1 |
| Mechanical ventilation | 8 (10) | 5 (4) | .1 |
| Resolution of PIV infection | 68/79 (86) | 96/108 (89) | .56 |
| Days from diagnosis of PIV infection to response, median (range) | 8 (1-92) | 13 (1-64) | .001 |
| Mortality within 30 days of PIV infection | 8/79 (10) | 8/108 (7) | .7 |
— indicates not applicable; and MDS, myelodysplastic syndrome and GVHD, graft-versus-host-disease.
Some data were missing, which may have changed the denominator.
A total of 37 microbiologically documented respiratory infections [19 bacterial infections, including Staphylococcus aureus (4), Stenotrophomonas maltophila (4), Pseudomonas sp (4), Mycobacterium avium complex (2), Enterococcus sp (2), Legionella pneumophila (1), Klebsiella pneumoniae (1), and Enterobacter cloacae (1); 13 viral infections, including respiratory syncytial virus (5), cytomegalovirus (4), herpes simplex virus (2), influenza B (1), and adenovirus (1); 4 fungal infections, including Aspergillus sp (3) and Rhizopus sp (1); and Pneumocystis jiroveci (1)] and 19 nonmicrobiologically documented infections.
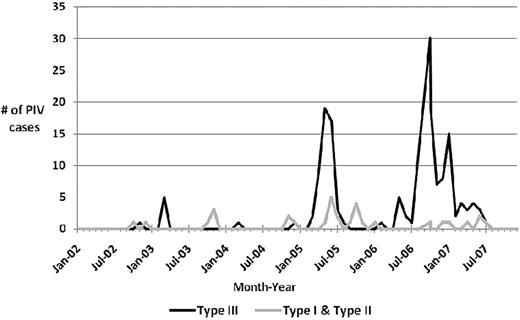
PIV type III was the most commonly identified virus (172 of 200, 86%), followed by PIV type I (22 of 200, 11%). During the study period, sporadic cases of PIV type I, II, and III occurred throughout the year, with seasonal outbreaks of PIV type I and II mainly occurring in the fall (Figure 1). However, seasonal outbreaks of PIV type III occurred mainly in the spring and summer, although the largest cluster of PIV type III cases occurred in the fall of 2006 in our cancer patients (Figure 1).
Patients with leukemia versus HSCT recipients
As shown in Table 1, patients with leukemia and HSCT recipients were comparable in sex, age, type of PIV, and site of PIV infection at presentation. There also were no statistically significant differences in the length of hospital or intensive care unit stay, rate of mechanical ventilation, or mortality rate within 30 days of PIV infection between the 2 groups (Table 1). On the other hand, patients with leukemia had a higher median APACHE II score at diagnosis and were more likely to be neutropenic and lymphopenic, to have progression to pneumonia, to be hospitalized because of PIV infection, and to have received chemotherapy within a month of diagnosis, compared with HSCT recipients (all P < .05).
Progression to pneumonia
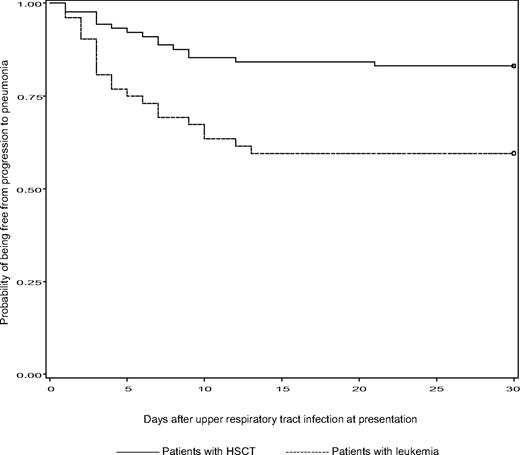
Patients with leukemia were more likely to progress to pneumonia than HSCT recipients during a 30-day period of follow-up after presentation with URI (P = .001; Figure 2). Compared with patients with URI, patients who progressed to pneumonia were more likely to be neutropenic and lymphopenic within a week of diagnosis until symptoms resolution, had a higher median APACHE II score, were more likely to be on chemotherapy and to have respiratory coinfections within a month of diagnosis of PIV infection, and were more likely treated with ribavirin and/or IVIG (all P < .05; Table 2). Multiple logistic regression analysis showed that 3 factors were significantly associated with progression to pneumonia: neutropenia, APACHE II score more than or equal to 15, and patients with respiratory coinfections within a month of PIV infection (Table 3).
Kaplan-Meier estimates of being free from progression to pneumonia between patients with leukemia and HSCT recipients during a 30-day period of follow-up after URI at presentation.P = .001 (log-rank test).
Kaplan-Meier estimates of being free from progression to pneumonia between patients with leukemia and HSCT recipients during a 30-day period of follow-up after URI at presentation.P = .001 (log-rank test).
Risk factors for progression to pneumonia
| Characteristic . | Patients with PIV pneumonia (n = 95), N (%) . | Patients with PIV URI (n = 105), N (%) . | Univariate analysis (P) . |
|---|---|---|---|
| Age ≥ 65 y | 19 (20) | 11 (10) | .06 |
| Relapse or refractory stage of underlying malignancy | 34/95 (36) | 26/104 (25) | .1 |
| Underlying condition | .002 | ||
| HSCT | 46 (48) | 74 (70) | |
| Leukemia | 49 (52) | 31 (30) | |
| GVHD | 22/46 (48) | 26/74 (35) | .17 |
| Type of PIV | |||
| Type I | 9 (9) | 13 (12) | .89 |
| Type II | 3 (3) | 3 (3) | |
| Type III | 83 (87) | 89 (85) | |
| Neutropenia (ANC < 500 μ/L) within a week of diagnosis to resolution of symptoms | 53/94 (56) | 22/102 (22) | < .0001 |
| Lymphopenia (ALC < 200 μ/L) within a week of diagnosis to resolution of symptoms | 62/94 (66) | 43/102 (42) | .001 |
| APACHE II score at diagnosis of PIV infection, median (range) | 13 (5-30) | 9 (3-20) | < .0001 |
| Steroid use | |||
| Corticosteroids | 52 (55) | 70 (67) | .08 |
| Prednisolone ≥ 600 mg | 35 (37) | 45 (43) | .39 |
| Chemotherapy within a month of diagnosis of PIV infection | 68 (72) | 44 (42) | < .0001 |
| Respiratory coinfections within a month of diagnosis of PIV infection | 41 (43) | 15 (14) | < .0001 |
| Therapy at URI stage | |||
| Aerosolized ribavirin and/or IVIG alone | 15/36 (42) | 22/105 (21) | .015 |
| Aerosolized ribavirin with or without IVIG | 9/36 (25) | 2/105 (2) | < .0001 |
| Characteristic . | Patients with PIV pneumonia (n = 95), N (%) . | Patients with PIV URI (n = 105), N (%) . | Univariate analysis (P) . |
|---|---|---|---|
| Age ≥ 65 y | 19 (20) | 11 (10) | .06 |
| Relapse or refractory stage of underlying malignancy | 34/95 (36) | 26/104 (25) | .1 |
| Underlying condition | .002 | ||
| HSCT | 46 (48) | 74 (70) | |
| Leukemia | 49 (52) | 31 (30) | |
| GVHD | 22/46 (48) | 26/74 (35) | .17 |
| Type of PIV | |||
| Type I | 9 (9) | 13 (12) | .89 |
| Type II | 3 (3) | 3 (3) | |
| Type III | 83 (87) | 89 (85) | |
| Neutropenia (ANC < 500 μ/L) within a week of diagnosis to resolution of symptoms | 53/94 (56) | 22/102 (22) | < .0001 |
| Lymphopenia (ALC < 200 μ/L) within a week of diagnosis to resolution of symptoms | 62/94 (66) | 43/102 (42) | .001 |
| APACHE II score at diagnosis of PIV infection, median (range) | 13 (5-30) | 9 (3-20) | < .0001 |
| Steroid use | |||
| Corticosteroids | 52 (55) | 70 (67) | .08 |
| Prednisolone ≥ 600 mg | 35 (37) | 45 (43) | .39 |
| Chemotherapy within a month of diagnosis of PIV infection | 68 (72) | 44 (42) | < .0001 |
| Respiratory coinfections within a month of diagnosis of PIV infection | 41 (43) | 15 (14) | < .0001 |
| Therapy at URI stage | |||
| Aerosolized ribavirin and/or IVIG alone | 15/36 (42) | 22/105 (21) | .015 |
| Aerosolized ribavirin with or without IVIG | 9/36 (25) | 2/105 (2) | < .0001 |
Multivariate logistic regression model for progression to pneumonia
| Variable . | N . | Odds ratio . | 95% CI . | P . |
|---|---|---|---|---|
| Neutropenia | < .001 | |||
| Yes | 56 | 4.3 | 1.9-10.0 | |
| No | 91 | 1.0 | ||
| APACHE II score | .016 | |||
| ≥ 15 | 33 | 3.5 | 1.3-9.8 | |
| < 15 | 114 | 1.0 | ||
| Respiratory coinfections within a month of PIV infection | < .0001 | |||
| Yes | 35 | 7.1 | 2.7-18.8 | |
| No | 112 | 1.0 |
| Variable . | N . | Odds ratio . | 95% CI . | P . |
|---|---|---|---|---|
| Neutropenia | < .001 | |||
| Yes | 56 | 4.3 | 1.9-10.0 | |
| No | 91 | 1.0 | ||
| APACHE II score | .016 | |||
| ≥ 15 | 33 | 3.5 | 1.3-9.8 | |
| < 15 | 114 | 1.0 | ||
| Respiratory coinfections within a month of PIV infection | < .0001 | |||
| Yes | 35 | 7.1 | 2.7-18.8 | |
| No | 112 | 1.0 |
Antiviral therapy
Nineteen patients received aerosolized ribavirin, with or without IVIG, and 36 patients received IVIG alone. The median time from the diagnosis of PIV infection to the start of either therapeutic modality was 3 days (range, 1-7 days). There were no statistically significant differences in the length of hospital stay, clinical response, duration of symptoms, and mortality rate among those who were treated with aerosolized ribavirin and/or IVIG and those who were treated with IVIG alone (data not shown). Interestingly, in patients with leukemia, the rate of progression to pneumonia was higher among patients treated at the URI stage with ribavirin, with or without IVIG, than those treated with IVIG alone (Table 4).
Effects of ribavirin and/or IVIG on progression to pneumonia and death
| Type of patients . | Outcomes . | Ribavirin (with/without IVIG) . | IVIG alone . | No treatment . | P . |
|---|---|---|---|---|---|
| Leukemia (n = 80) | n = 9 | n = 6 | n = 69 | ||
| Progression to pneumonia in patients treated at URI stage, N (%) | 6/6 (100) | 1/5 (20) | 42/69 (61) | .02* | |
| Death within 30 days of follow-up, N (%) | 1/9 (11) | 0/6 (0) | 7/65 (11) | > .99 | |
| HSCT (n = 120) | n = 10 | n = 30 | n = 80 | ||
| Progression to pneumonia in patients treated at URI stage, N (%) | 3/5 (60) | 5/21 (24) | 38/94 (40) | .27 | |
| Death within 30 days of follow-up, N (%) | 1/10 (10) | 3/29 (10) | 4/69 (6) | .52 |
| Type of patients . | Outcomes . | Ribavirin (with/without IVIG) . | IVIG alone . | No treatment . | P . |
|---|---|---|---|---|---|
| Leukemia (n = 80) | n = 9 | n = 6 | n = 69 | ||
| Progression to pneumonia in patients treated at URI stage, N (%) | 6/6 (100) | 1/5 (20) | 42/69 (61) | .02* | |
| Death within 30 days of follow-up, N (%) | 1/9 (11) | 0/6 (0) | 7/65 (11) | > .99 | |
| HSCT (n = 120) | n = 10 | n = 30 | n = 80 | ||
| Progression to pneumonia in patients treated at URI stage, N (%) | 3/5 (60) | 5/21 (24) | 38/94 (40) | .27 | |
| Death within 30 days of follow-up, N (%) | 1/10 (10) | 3/29 (10) | 4/69 (6) | .52 |
The only significant difference was between patients treated with ribavirin versus IVIG (P = .02).
Mortality
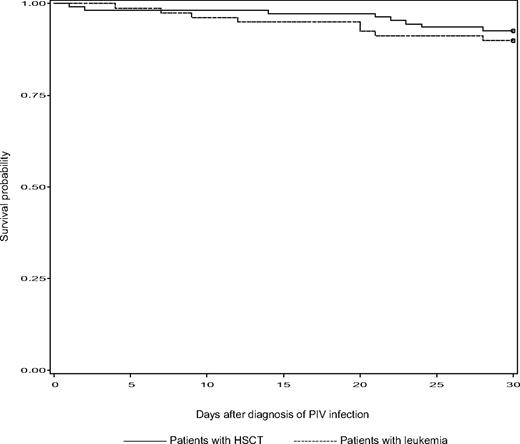
The mortality rate at day 30 was 10% (8 of 79) in patients with leukemia and 7% (8 of 108) in HSCT recipients (P = .7). Among patients who presented with or progressed to pneumonia, the mortality rate was higher, at 17%. There was no significant difference in survival within 30 days after diagnosis of PIV infection between patients with leukemia and HSCT recipients (P = .5; Figure 3). The cause of death was acute respiratory failure and/or multiorgan failure in 13 patients. Compared with patients who were alive at 30 days after diagnosis of PIV infection, patients who had died were more likely to have had a relapsed or refractory underlying malignancy, neutropenia within a week of diagnosis until resolution of symptoms, a higher median APACHE II score at diagnosis, and respiratory coinfections within a month of diagnosis (all P < .05; Table 5). Multiple logistic regression analysis showed that 3 factors were independently associated with death within 30 days of diagnosis of PIV infection: cancer status, APACHE II score, and corticosteroid usage at more than or equal to 600 mg of prednisolone or equivalent (Table 6).
Kaplan-Meier estimates of survival between patients with leukemia and HSCT recipients during a 30-day period of follow-up after diagnosis of PIV infection.P = .5 (log-rank test).
Kaplan-Meier estimates of survival between patients with leukemia and HSCT recipients during a 30-day period of follow-up after diagnosis of PIV infection.P = .5 (log-rank test).
Outcome of patients with PIV infection within 30 days of diagnosis
| Characteristic . | Patients who died within 30 days of PIV infection (n = 16), N (%) . | Patients who survived > 30 days of PIV infection (n = 171), N (%) . | Univariate analysis (P) . |
|---|---|---|---|
| Age ≥ 65 y | 4 (25) | 24 (14) | .27 |
| Relapse or refractory stage of underlying malignancy | 11/16 (69) | 48/170 (28) | .0009 |
| HSCT recipients | 8 (50) | 100 (58) | .51 |
| GVHD | 4/8 (50) | 38/100 (38) | .71 |
| Neutropenia (ANC < 500 μ/L) within a week of diagnosis to resolution of symptoms | 11/16 (69) | 64/167 (38) | .018 |
| Lymphopenia (ALC < 200 μ/L) within a week of diagnosis to resolution of symptoms | 12/16 (75) | 90/167 (54) | .1 |
| APACHE II score at diagnosis, median (range) | 20 (9-30) | 11 (3-24) | < .0001 |
| Steroid use | |||
| Corticosteroids | 9 (56) | 103 (60) | .76 |
| Prednisolone ≥ 600 mg | 8 (50) | 63 (37) | .3 |
| Chemotherapy within a month of diagnosis of PIV infection | 12 (75) | 96 (56) | .14 |
| Respiratory coinfections within a month of diagnosis of PIV infection | 9 (56) | 47 (27) | .023 |
| Antiviral therapy | 5 (31) | 48 (28) | .78 |
| Aerosolized ribavirin with or without IVIG | 2 (13) | 17 (10) | .67 |
| IVIG with or without ribavirin | 5 (31) | 38 (22) | .53 |
| Characteristic . | Patients who died within 30 days of PIV infection (n = 16), N (%) . | Patients who survived > 30 days of PIV infection (n = 171), N (%) . | Univariate analysis (P) . |
|---|---|---|---|
| Age ≥ 65 y | 4 (25) | 24 (14) | .27 |
| Relapse or refractory stage of underlying malignancy | 11/16 (69) | 48/170 (28) | .0009 |
| HSCT recipients | 8 (50) | 100 (58) | .51 |
| GVHD | 4/8 (50) | 38/100 (38) | .71 |
| Neutropenia (ANC < 500 μ/L) within a week of diagnosis to resolution of symptoms | 11/16 (69) | 64/167 (38) | .018 |
| Lymphopenia (ALC < 200 μ/L) within a week of diagnosis to resolution of symptoms | 12/16 (75) | 90/167 (54) | .1 |
| APACHE II score at diagnosis, median (range) | 20 (9-30) | 11 (3-24) | < .0001 |
| Steroid use | |||
| Corticosteroids | 9 (56) | 103 (60) | .76 |
| Prednisolone ≥ 600 mg | 8 (50) | 63 (37) | .3 |
| Chemotherapy within a month of diagnosis of PIV infection | 12 (75) | 96 (56) | .14 |
| Respiratory coinfections within a month of diagnosis of PIV infection | 9 (56) | 47 (27) | .023 |
| Antiviral therapy | 5 (31) | 48 (28) | .78 |
| Aerosolized ribavirin with or without IVIG | 2 (13) | 17 (10) | .67 |
| IVIG with or without ribavirin | 5 (31) | 38 (22) | .53 |
Multivariate logistic regression model for death within 30 days of diagnosis
| Variable . | N . | Odds ratio . | 95% CI . | P . |
|---|---|---|---|---|
| Cancer status | .028 | |||
| Relapse or refractory | 56 | 5.0 | 1.2-20.8 | |
| Remission or active | 123 | 1.0 | ||
| APACHE II score | < .001 | |||
| ≥ 15 | 40 | 18.9 | 4.1-86.9 | |
| < 15 | 139 | 1.0 | ||
| Use of steroids | .028 | |||
| ≥ 600 mg prednisolone or equivalent | 68 | 5.5 | 1.2-25.3 | |
| < 600 mg of prednisolone or no steroids used | 111 | 1.0 |
| Variable . | N . | Odds ratio . | 95% CI . | P . |
|---|---|---|---|---|
| Cancer status | .028 | |||
| Relapse or refractory | 56 | 5.0 | 1.2-20.8 | |
| Remission or active | 123 | 1.0 | ||
| APACHE II score | < .001 | |||
| ≥ 15 | 40 | 18.9 | 4.1-86.9 | |
| < 15 | 139 | 1.0 | ||
| Use of steroids | .028 | |||
| ≥ 600 mg prednisolone or equivalent | 68 | 5.5 | 1.2-25.3 | |
| < 600 mg of prednisolone or no steroids used | 111 | 1.0 |
Discussion
To our knowledge, this is the only study to date to exclusively evaluate and compare PIV infection in a large number of patients with leukemia and recipients of HSCT. Our data show that almost half of the patients progressed to PIV pneumonia, with a subsequent mortality rate of 17%. This confirms that PIV infection causes significant morbidity and mortality not only in patients after HSCT, as described in previous studies,1,4,10,14,15,18,19 but among patients with leukemia as well.
PIV type III was the most commonly isolated virus in all patients. Several other studies have documented a high occurrence of PIV type III infection in HSCT recipients.1,3-5,19,20 Most of the outbreaks of PIV type III infection occurred in spring and summer, as has been described by other studies.4,10,21
Currently, published data on PIV infection in patients with leukemia are limited. A recently published study by Srinivasan et al found that after influenza (A and B), PIV was the second most common respiratory viral infection among children with hematologic malignancies.22 In the same study, they also found that URI was more common than LRI among these children, with no mortality attributed to PIV infection.22 Earlier studies from our institution involving 47 leukemic patients5 and 61 HSCT recipients10 have reported progression to pneumonia in 55% and 44% of the patients and associated mortality rates of 27% and 37%, respectively. Our study shows almost the same rate of progression to pneumonia in patients with leukemia (61%) and in HSCT recipients (39%) but with lower frequency in the latter. However, a multiple logistic regression analysis did not reveal underlying condition (leukemia vs HSCT) as a risk factor for progression to pneumonia. Although more patients with leukemia were neutropenic, lymphopenic, hospitalized because of PIV infection, progressed to pneumonia, and had higher APACHE II scores at presentation, the mortality rate within 30 days of infection was comparable between the 2 groups.
Our study reports an associated mortality rate of 17% among patients who had PIV pneumonia. This rate was consistent between patients with leukemia and HSCT recipients (16% vs 17%, respectively) but is much lower than rates previously reported in patients with PIV pneumonia (range, 25%-66%).1,3-5,10 This could be explained in part by better supportive care for these patients in recent years. Multiple risk factors for death were identified in a logistic regression model, including the receipt of high-dose steroids, having an APACHE II score more than or equal to 15, and relapsed or refractory stage of malignancy at diagnosis of PIV infection. Interestingly, being on high-dose steroids at the time of diagnosis was not associated with an increased likelihood of progression to pneumonia, as reported by Nichols et al in recipients of HSCT,4 but was associated with a higher mortality rate. As suggested in the previous study,4 tapering of steroid use, the only modifiable risk factor, at the time of diagnosis of PIV infection may need to be considered, as it may improve overall outcome.
The significant morbidity and mortality associated with PIV pneumonia in these immunocompromised patients underscore the need for effective therapy. Because of its in vitro activity against PIV, ribavirin, mainly in its aerosolized form, has been used to treat this infection, with mixed results.1,4,5,10,15-17 In our study, data on therapy should be interpreted with caution as we could have missed additional confounders that may have affected outcomes. Nevertheless, treatment with aerosolized ribavirin and/or IVIG did not prevent progression to pneumonia and did not affect duration of illness or survival in either group. This is consistent with earlier studies.4,18,19 The significant proportion of progression to pneumonia in our patients with leukemia treated with aerosolized ribavirin with or without IVIG versus IVIG alone (Table 4) is more likely the result of selection bias (as sicker patients are more likely to be treated with ribavirin) and to the small number of patients treated. In addition, mortality within 30 days of PIV infection was almost the same between treated versus untreated patients in both groups. Based on these data, we seldom recommend aerosolized ribavirin for therapy of PIV infection at our institution, but if patients progress to pneumonia, we may use IVIG, along with intensification of the antimicrobial regimen for possible superimposed bacterial and/or fungal infections.
A significant number of patients with PIV infection had respiratory copathogens isolated from the bronchoalveolar lavages or nasopharyngeal washings. Some of the earlier studies reported a high incidence of aspergillosis among patients with PIV type III pneumonia.16,23 However, Aspergillus spp was isolated from only 3 patients in our study, whereas 1 patient had an invasive Rhizopus infection. Most of the respiratory coinfections were bacterial in etiology (Pseudomonas aeruginosa, Staphylococcus aureus, and Stenotrophomonas maltophilia), whereas some were viral (respiratory syncytial virus and cytomegalovirus). These dual-microbial or polymicrobial infections are not uncommon in immunocompromised patients. However, it raises the question of a possible association, as seen with the influenza virus, between PIV infection and superimposed bacterial, viral, or fungal infections. As suggested by Nichols et al,16 direct damage to the respiratory epithelium from PIV replication may predispose patients to these superimposed infections by facilitating the penetration of various organisms.
One of the major limitations of this study is its retrospective nature. Because all the information was exclusively obtained from the available medical records, there is a chance that important information could have been missed. On the other hand, some patients with mild symptoms may not have sought medical attention; thus, their infections may have resolved on their own, which may also have contributed to selection bias.
In conclusion, our study shows that PIV infection is the cause of significant morbidity and mortality, not only in recipients of HSCT but also in patients with leukemia. Multiple unmodifiable risk factors are independently associated with progression to pneumonia and mortality. Aerosolized ribavirin, with or without IVIG, did not appear to improve the duration of illness, length of hospitalization, or survival of patients with leukemia and recipients of HSCT. Because existing therapeutic options are inadequate, infection control strategies continue to be the cornerstone for preventing the spread of this infection among susceptible patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr David Hewett-Emmett for his valuable contributions of ideas, comments, and assistance in undertaking and writing of the research summarized here.
Authorship
Contribution: R.F.C. and S.S.H. designed the study; R.F.C., S.S.H., D.B.R., S.S.G., A.D., and A.M.K. performed the clinical research and controlled and analyzed data; Y.J. performed the statistical analysis; S.S.H., D.B.R., S.S.G., and R.F.C. wrote the paper; and all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roy F. Chemaly, Department of Infectious Diseases, Infection Control, and Employee Health, Unit 1460, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030-4009; e-mail: rfchemaly@mdanderson.org.