Abstract
We discovered that miR-27b controls 2 critical vascular functions: it turns the angiogenic switch on by promoting endothelial tip cell fate and sprouting and it promotes venous differentiation. We have identified its targets, a Notch ligand Delta-like ligand 4 (Dll4) and Sprouty homologue 2 (Spry2). miR-27b knockdown in zebrafish and mouse tissues severely impaired vessel sprouting and filopodia formation. Moreover, miR-27b was necessary for the formation of the first embryonic vein in fish and controlled the expression of arterial and venous markers in human endothelium, including Ephrin B2 (EphB2), EphB4, FMS-related tyrosine kinase 1 (Flt1), and Flt4. In zebrafish, Dll4 inhibition caused increased sprouting and longer intersegmental vessels and exacerbated tip cell migration. Blocking Spry2 caused premature vessel branching. In contrast, Spry2 overexpression eliminated the tip cell branching in the intersegmental vessels. Blockade of Dll4 and Spry2 disrupted arterial specification and augmented the expression of venous markers. Blocking either Spry2 or Dll4 rescued the miR-27b knockdown phenotype in zebrafish and in mouse vascular explants, pointing to essential roles of these targets downstream of miR-27b. Our study identifies critical role of miR-27b in the control of endothelial tip cell fate, branching, and venous specification and determines Spry2 and Dll4 as its essential targets.
Introduction
Angiogenic balance and endothelial cell fate are determined by the extracellular signals generated by angiogenic growth factors (stimuli) and inhibitors.1,2 Molecular mechanisms that determine angiogenic balance have been extensively studied; however, our understanding of the key intracellular events remains incomplete. Recent studies have shown that growing vasculature follows the gradients of VEGF, which are sensed by the nonproliferative endothelial tip cells that direct further expansion of the vascular sprout. The density and morphology of the growing vasculature is dictated by the frequency of tip cells. Following behind tip cells, proliferating stalk cells ensure sprout lengthening and lumen formation. Their fate is maintained by Delta like ligand 4 (Dll4), which is produced by the tip cells. Dll4 binds Notch on adjacent stalk cells, and the resulting signal represses tip fate and ensures proliferation and sprout lengthening toward the VEGF source.3 Stalk cell proliferation and neovessel integrity depend on VEGF and other pro-angiogenic cytokines, such as basic fibroblast growth factor, which through cognate receptors activate mitogenic kinases converging on Erk1/2.4 In normal tissues, VEGF release from the extracellular matrix is tightly controlled and improper VEGF gradients cause abnormally high numbers of tip cells and aberrant vascular patterns.5,6
A large family of Sprouty (Spry) genes regulates secondary branching of the tubular structures in the kidney, lung, and ear.7 This family encodes proteins Spry1 through 4 and sprouty-related domain 1 (SPRED1) and SPRED2. In the cells, Spry proteins attenuate MAP kinase activation by the epidermal growth factor, fibroblast growth factors 7 and 10, and glial-derived neurotropic factor by blocking phospholipase Cγ, Ras-GTP, or Raf-1.8 Studies of knock-out mice show that Spry4 and Spry2 block physiologic angiogenesis.9-11
To date, the effects of natural angiogenesis inhibitors on tip cell fate and branching remain unknown. Although they reduce MAP kinase activation by angiogenic stimuli, the underlying mechanisms are not always clear.12 The results of the present study show that angiogenesis inhibitors interfere with vascular sprouting and oppose tip cell fate by blocking miR-27b expression. miRNAs are recognized as major temporal and spatial regulators of gene expression.13 They act posttranscriptionally through binding to the 3′ untranslated regions of the target mRNA; perfect base pairing results in mRNA degradation and partial mismatch causes abortive translation.13-15 The link between the miRNA and angiogenesis has been established using knockdown of the miRNA processing enzyme Dicer in cultured endothelial cells16 and in mice homozygous for a hypomorphic Dicer allele.17 These and further studies identified a subset of miRNAs involved in the regulation of angiogenesis. Most of them, such as let-7f, miR-27b, miR-126, and hypoxia-dependent miR-210, are pro-angiogenic. In contrast, miR-214, miR-221, and miR-222 suppress angiogenesis.18,19
The effects of miR-126 and miR-210 have been studied in detail. Endothelial-specific miR-126 is required for normal vascular development and angiogenic response.20,21 Three of its targets, VCAM-1, SPRED1, and the p85 PI3K subunit, contribute critically to endothelial cell adhesion, proliferation, and integrity. Hypoxia-driven miR-210 promotes angiogenesis by blocking Ephrin A3 (EphA3).22 Conversely, anti-angiogenic miR-221/222 act by blocking endothelial c-Kit expression.23
In the present study, we show that a natural angiogenesis inhibitor pigment epithelial-derived factor (PEDF) down-regulates miR-27b in the activated endothelial cells. We demonstrate that miR-27b is required for angiogenesis and that it acts by blocking the expression of Dll4 and Spry2. Blocking miR-27b in zebrafish embryo inhibited sprouting of the intersegmental vessels (ISVs) from the dorsal aorta (DA) and the emergence of T-shaped tip cells necessary for the formation of dorsal longitudinal anastomotic vessels (DLAVs). Similarly, in mouse aorta, the blockade of miR-27b impaired sprouting. Conversely, miR-27b knockdown reduced branching and significantly increased the diameter of tumor vessels in a syngeneic mouse model. Interestingly, miR-27b knockdown hampered formation of the first embryonic vein in zebrafish and expression of venous markers in cultured human endothelium and in vivo. Dll4 knockdown exacerbated branching and caused emergence of vessel-free, hyper-migratory endothelial cells. Spry2 overexpression also blocked the occurrence of T-shaped ISV tip cells. In contrast, Spry2 knock-out in mouse vascular explants and in zebrafish enhanced angiogenic sprouting and rescued the miR-27b knockdown phenotype. Furthermore, miR-27b controlled arterial-venous segregation in the zebrafish embryo, whereby Dll4, via Notch and EphB2, promoted arterial fate and Spry2 supported expression of Flt4 (VEGFR3), which is necessary to maintain arterial fate.24,25 Defective segregation in miR-27b morphants was rescued by knockdown of either Dll4 or Spry2. Our results for the first time reveal a specific function of miR-27b in vascular development and identify it as a critical modulator of angiogenic balance regulated by angiogenic inhibitors and stimuli, which controls the angiogenic switch by promoting tip cell fate and capillary branching. We also identify a previously unknown function for Spry2: the control of venous specification via EphB4 and Flt4.
Methods
Cell lines and reagents
For details on cell lines and reagents, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
miRNA expression microarrays
miRNA expression microarrays were performed by LC Sciences (Houston, TX). Total RNA samples labeled with Cy-3 and Cy-5 dyes for dual sample analyses were hybridized with the probe chip and signal measured after background subtraction/normalization. Data are available at the Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo) under accession number GSE34735.
RT-PCR
Total RNA was extracted with the miRCURY RNA isolation kit (Exiqon). miR-27b and miR-126 were measured with the miRCURY LNA miRNA PCR System.
Northern blot analysis
Total RNA extracted with TRIzol reagent (Sigma-Aldrich) and 5-μg aliquots were run on 15% denaturing PAAG (Bio-Rad). The RNA was transferred to a nylon membrane (Whatman Nytran Nylon N Charged membrane; Sigma-Aldrich) and hybridized with miRCURY LNA probes labeled with 3′-digoxigenin (Exiqon).
In situ hybridization of whole-mount zebrafish embryos
In situ hybridization was performed as described previously.26 Briefly, dechorionated embryos were fixed in 4% paraformaldehyde, permeabilized, and digested with proteinase K. For details and probes, see supplemental Methods.
Western blot analysis
Cell extracts prepared with M-PER reagent (Thermo Fisher) were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, probed with primary Abs and developed with HRP-conjugated secondary Abs. Abs are listed in supplemental Methods.
Constructs
Constructs encoding hsa-miR-27b precursor and scrambled control Spry2 and Dll4 3′-untranslated region (3′-UTR) and mutated 3′-UTR reporters were from Genecopoeia. The Spry2 zebrafish ortholog from Genecopoeia was cloned in pGEM-7Zf(+) (Promega).
Luciferase reporter assay
HEK 293T cells were transfected with 3′-UTR reporter, mutated 3′-UTR reporter, and miR-27b precursor expression constructs (Lipofectamine 2000; Invitrogen) and the assay was performed with the dual luciferase reporter assay system (Promega).
MOs
Targeting and control morpholinos (MOs; supplemental Table 1) and Endo-Porter reagent were from Gene Tools.
Experimental animals
Athymic nude mice (hsd:athymic nude-foxn1; Harlan Laboratories) and Spry2+/− mice27 on the Balb/c background were kept at Northwestern University Center for Comparative Medicine in accordance with the National Institutes of Health guidelines and protocols approved by Northwestern University Animal Care and Use Committee. Transgenic Tg(fli1a:EGFP)y1 zebrafish harboring the fli1α:EGFP reporter on “sheer” or on wild-type background (Dr H. Tomasiewicz, University of Wisconsin, Milwaukee, WI, and the Zebrafish International Resource Center, Eugene, OR) were maintained according to the National Institutes of Health guidelines and the protocols approved by Northwestern University and CMRC Animal Care and Use Committee (for details, see supplemental Methods).
Tumor implantation
Athymic nude mice received subcutaneous injections of 106 Lewis lung carcinoma (LLC) cells in 0.1 mL of PBS in the flank. Vivo-MO-miR27b (12.5 mg/kg daily) was injected into tumors (5-7 mm in diameter) for 5 days, after which time the tumors were harvested.
Aortic ring assay
Abdominal aortas from Spry2+/+, Spry2+/−, and Spry2−/− mice were excised, cut into 1-mm rings, and embedded in Matrigel (BD Biosciences). Bovine brain extract (BBE; 200 μg/mL) was used to induce sprouting. MOs were added at 10μM, as indicated.
Microinjections
MOs in sterile water (5-18 μg/μL, 1.5 nL) were injected in fertilized eggs at the 1- to 2-cell stage with the Pneumatic PicoPump (World Precision Instruments).
Immunostaining
Tumor sections (5 μm) were fixed and probed with CD31 rat anti–mouse antibody followed by donkey anti–rat Rhodamine Red-X conjugate (Jackson ImmunoResearch) to detect blood vessels. Nuclei were highlighted with 4′,6-diamidine-2-phenyl indole. To assess proliferation, sections were stained with rabbit anti–mouse Ki-67 antibody (Santa Cruz Biotechnology), followed by FITC-conjugated goat anti–rabbit Abs (Jackson ImmunoResearch).
Microscopy
Tumor sections were viewed under a Nikon Diaphot 2000 fluorescent microscope with a 20× objective and digital images were taken for permanent record. Zebrafish embryos (21-51 hours postfertilization [hpf]) were viewed under a stereo zoom microscope (Discovery V8). Serial images were taken and assembled with Image-Pro Plus Version 4 (Media Cybernetics) and Corel Photo Paint × 5. Confocal images were collected with a Zeiss LSM510 microscope. Aortic rings were imaged with Discovery V8 stereo zoom (bright-light microscopy).
Morphometry and statistical analysis
Images were analyzed with MetaMorph software (Molecular Devices). All quantitative traits were compared pairwise and statistical significance was determined by a 1-tailed Student t test. P < .05 was considered significant.
Results
PEDF attenuates miR-27b in VEGF-stimulated endothelium
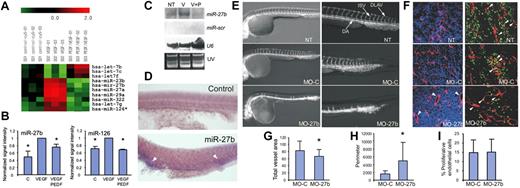
Using miRNA arrays, we compared miRNA expression profiles in control, untreated human microvascular endothelial cells and in those treated with VEGF alone and in the presence of PEDF. Using a 1.5-fold cutoff, we selected miRNA (Figure 1A) with levels significantly decreased by PEDF (P < .05), including human miR23b, miR-27a, miR-27b, miR-29a, miR-126, and let-7g. Using real-time RT-PCR and Northern blot, we confirmed the changes in miR-126 and miR-27b (Figure 1B-C). miR-126 has been linked previously with angiogenesis20,21 and served as internal control for our data. miR-27b participation in angiogenesis was also shown previously16,18,20,28 ; however, its cellular functions and molecular mechanisms are not fully understood.
miR-27b controls vascular sprouting. (A) RNA was isolated from human microvascular endothelial cells grown in control medium (C, control), treated with VEGF (1-10 ng/mL) or VEGF + PEDF (10nM) and subjected to miRNA array analysis. A fragment of miRNA array shows the opposing effect of VEGF and PEDF on the expression of select miRNA (P < .05 by the 1-tailed Student t test). (B) Real-time RT-PCR was performed with primers for miR-27b (left panel) and miR-126 (right panel), with Let-7a as an internal control. (C) Northern blot was probed for indicated miRNA or U6 RNA to assess loading. The UV image is shown. (D) Whole-mount in situ hybridization for pri-miR-27b. The sense strand probe was used as a negative control (top panel). (E) Fertilized Tg(fli1a:EGFP)y1 zebrafish eggs were injected with MOs against miR-27b (MO-27b), control MOs (MO-C), or left untreated (NT). The embryos were photographed at 24 hpf. (F) Mice with subcutaneous LLC tumor xenografts (5-7 mm in diameter) were treated with daily intratumoral injections of MO-C or MO-27b (12.5 mg/kg). After 5 days, the tumors were excised, sectioned, and stained for the endothelial marker CD31 (red) and the proliferation marker Ki67 (green). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). Arrowheads indicate proliferating endothelial cells. (G-I) Experiments shown in panel F were subjected to morphometric analysis with MetaMorph software. *P < .03 (G) and P < .003 (H). No significant difference was noted in panel I, with P = .47 by the 1-tailed Student t test.
miR-27b controls vascular sprouting. (A) RNA was isolated from human microvascular endothelial cells grown in control medium (C, control), treated with VEGF (1-10 ng/mL) or VEGF + PEDF (10nM) and subjected to miRNA array analysis. A fragment of miRNA array shows the opposing effect of VEGF and PEDF on the expression of select miRNA (P < .05 by the 1-tailed Student t test). (B) Real-time RT-PCR was performed with primers for miR-27b (left panel) and miR-126 (right panel), with Let-7a as an internal control. (C) Northern blot was probed for indicated miRNA or U6 RNA to assess loading. The UV image is shown. (D) Whole-mount in situ hybridization for pri-miR-27b. The sense strand probe was used as a negative control (top panel). (E) Fertilized Tg(fli1a:EGFP)y1 zebrafish eggs were injected with MOs against miR-27b (MO-27b), control MOs (MO-C), or left untreated (NT). The embryos were photographed at 24 hpf. (F) Mice with subcutaneous LLC tumor xenografts (5-7 mm in diameter) were treated with daily intratumoral injections of MO-C or MO-27b (12.5 mg/kg). After 5 days, the tumors were excised, sectioned, and stained for the endothelial marker CD31 (red) and the proliferation marker Ki67 (green). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). Arrowheads indicate proliferating endothelial cells. (G-I) Experiments shown in panel F were subjected to morphometric analysis with MetaMorph software. *P < .03 (G) and P < .003 (H). No significant difference was noted in panel I, with P = .47 by the 1-tailed Student t test.
miR-27b is required for capillary branching/sprouting
Seeking the role of miR-27b in vivo, we used Danio rerio (zebrafish) and LLC mouse flank tumors to model developmental and tumor angiogenesis. We and others have established miR-27b expression in human and mouse endothelium28,29 (Figure 1B-C). In situ hybridization in zebrafish showed expression in the areas overlapping with the vasculature at 24 hpf (Figure 1D). Injection of MOs against miR-27b (MO-27b) in fertilized zebrafish eggs at the 1- to 2-cell stage caused dramatic impairment of angiogenesis at 24 and 48 hpf, whereas control MOs (MO-C) had no effect (Figure 1E). Sprouting of the ISVs from the DA and formation of DLAVs were severely compromised by MO-27b. In contrast, in murine LLC tumors, MO-27b caused only modest, albeit significant decrease in microvascular density (Figure 1F-G; P < .04). However, MO-27b drastically altered the morphology of the tumor vasculature, promoting large open vessels instead of nonproductive tumor capillaries (Figure 1F). Morphometric analysis revealed a statistically significant 3-fold increase in vessel circumference when miR-27b was inhibited, whereas control MOs had no effect (Figure 1H; P < .004). Staining for the proliferation marker Ki67 revealed similar mitotic rates in CD31-positive endothelium in the LLC tumors treated with MO-27b or MO-C (Figure 1F and I; P > .46), suggesting unimpaired proliferation combined with the loss of branching/sprouting. The down-regulation of miR-27b in the tumors was confirmed by increased expression of its targets (supplemental Figure 1).
miR-27b targets Dll4 and Spry2 in the vascular endothelium
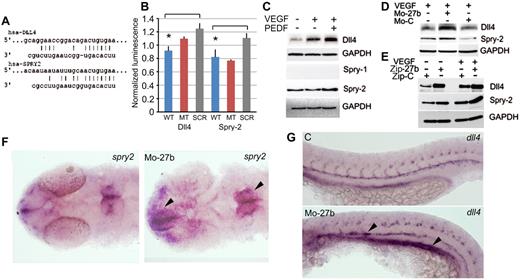
Seeking miR-27b putative targets relevant for branching or sprouting, we identified Dll4 and Spry2, which have been implicated in vascular guidance and branching of the tubular structures, respectively.7,11 Analysis of their 3′-UTRs revealed high-affinity binding sites for miR-27b at positions 144-150 of Dll4 mRNA and 381-387 of Spry2 mRNA (Figure 2A). We coexpressed miR-27b precursor, scrambled control, and constructs encoding wild-type and mutant Dll4 and Spry2 3′-UTRs fused to luciferase reporter. We detected statistically significant decreases in luciferase activity with wild-type miR-27b compared with the scrambled miR-27b control (Figure 2B; P < .002) for both Dll4 and Spry2 UTRs, suggesting miR-27b binding and repression of the respective genes. Western blot analysis revealed elevated Dll4 and Spry2 in the endothelial cells treated with the combination of VEGF and PEDF compared with VEGF-treated controls; Spry1 was not detectable (Figure 2C and supplemental Figure 1A). PEDF alone caused no significant change in either Dll4 or Spry-2 (supplemental Figure 1B). In VEGF-stimulated ECs, PEDF increased Notch activation, as evidenced by increased Notch intracellular domain (supplemental Figure 1C), likely caused by increased Dll4. Moreover, MO-27b and lentivirally expressed miRZip-27b miRNA inhibitor dramatically increased Dll4 and Spry2 levels in VEGF-stimulated endothelial cells, whereas MO-C and control lentivirus had no effect (Figure 2D-E). In zebrafish, miR-27b knockdown noticeably increased dll4 and spry2 expression (Figure 2F-G). Similarly, Dll4 and Spry2 were up-regulated in mouse LLC tumors treated with MO-27b compared with MO-C (supplemental Figure 2).
miR-27b targets Dll4 and Spry2. (A) Putative miR-27b binding sequences in Dll4 and Spry2 3′UTRs. (B) 3′UTR reporter assay. Reporter constructs with luciferase reporter fused to Dll4 and Spry2 3′UTR sequences, wild-type or mutated (MT), were cotransfected into HEK-293T cells together with vectors encoding miR-27b precursor, wild-type or scrambled (SCR). Luciferase activity was measured after 24 hours and normalized to Renilla luciferase control. *Statistically significant differences from SCR and/or MT controls by the 1-tailed Student t test. (C) Human microvascular endothelial cells were treated as indicated and Western blots probed with the Abs for Dll4, Spry1, Spry2, and GAPDH (loading control). (D-E) HUVECs were treated with MO-27b, MO-C, or transfected with lentiviral miRNA inhibitor Zip-27b or Zip-C control. Dll4 and Spry2 were measured by Western blot. GAPDH was used as loading control. (F-G) Whole-mount in situ hybridization with spry2 (F) and dll4 probes (G).
miR-27b targets Dll4 and Spry2. (A) Putative miR-27b binding sequences in Dll4 and Spry2 3′UTRs. (B) 3′UTR reporter assay. Reporter constructs with luciferase reporter fused to Dll4 and Spry2 3′UTR sequences, wild-type or mutated (MT), were cotransfected into HEK-293T cells together with vectors encoding miR-27b precursor, wild-type or scrambled (SCR). Luciferase activity was measured after 24 hours and normalized to Renilla luciferase control. *Statistically significant differences from SCR and/or MT controls by the 1-tailed Student t test. (C) Human microvascular endothelial cells were treated as indicated and Western blots probed with the Abs for Dll4, Spry1, Spry2, and GAPDH (loading control). (D-E) HUVECs were treated with MO-27b, MO-C, or transfected with lentiviral miRNA inhibitor Zip-27b or Zip-C control. Dll4 and Spry2 were measured by Western blot. GAPDH was used as loading control. (F-G) Whole-mount in situ hybridization with spry2 (F) and dll4 probes (G).
miR-27b promotes angiogenesis and tip cell fate by blocking Dll4
Dll4, a Notch ligand, controls vascular branching by repressing tip cell fate.30 In agreement with earlier studies,24 we observed aberrant branching in Dll4 (MO-Dll) morphants at 30 hours compared with controls (Figure 3A MO-Dll4 [2] arrowheads), increased cell numbers in the ISVs (Figure 3A-B), and augmented filopodia formation (Figure 3A,C). In addition, in Dll4 morphants, we observed multiple endothelial cells lacking connection to specific ISV (Figure 3A MO-Dll4 [1] arrowheads and supplemental Figure 3). This lack of continuity likely reflects excessive motility when tip cell fate is not restricted by Dll4. Combining MO-27b with MO-Dll4 largely rescued defective sprouting seen in the individual miR-27b and Dll4 morphants (Figure 3A-C). It is thought that Flt1 (VEGFR1) acts as a decoy for VEGFR2 (Kdrl in zebrafish). Flt1 is relatively low in the endothelial tip cells, and Dll4, via Notch, promotes its expression in the stalk cells.31,32 In agreement, Flt1 expression was higher in miR-27b morphants than in control embryos. Dll4 knockdown in the double morphants reversed Flt-1 expression to near wild-type levels (Figure 3D and supplemental Figure 4).
miR-27b controls tip cell fate via Dll4. (A) Representative images of zebrafish vasculature in miR-27b, Dll4, and double miR-27b/Dll4 morphants. Arrowheads indicate disconnected, hyper-migratory tip cells (MO-Dll4 [1]) and excessive branching (MO-Dll4 [2]) in Dll4 morphants. Note decreased sprout length, lack of branched tip cells, and decreased filopodia formation in miR-27b morphants and restored phenotype in double morphants. Broken line indicates that the image is a composite of 2 independent photomicrographs of the same field taken in different focal planes (B-C). Quantitative analysis of experiment shown in panel A. *P < .0002 and **P < .00007 between control and miR-27b knockdown. (D) Expression of flt1 in miR-27b, Dll4, and double morphants. Note increased Flt1 mRNA on miR-27 knockdown and normalized phenotype of the double morphants.
miR-27b controls tip cell fate via Dll4. (A) Representative images of zebrafish vasculature in miR-27b, Dll4, and double miR-27b/Dll4 morphants. Arrowheads indicate disconnected, hyper-migratory tip cells (MO-Dll4 [1]) and excessive branching (MO-Dll4 [2]) in Dll4 morphants. Note decreased sprout length, lack of branched tip cells, and decreased filopodia formation in miR-27b morphants and restored phenotype in double morphants. Broken line indicates that the image is a composite of 2 independent photomicrographs of the same field taken in different focal planes (B-C). Quantitative analysis of experiment shown in panel A. *P < .0002 and **P < .00007 between control and miR-27b knockdown. (D) Expression of flt1 in miR-27b, Dll4, and double morphants. Note increased Flt1 mRNA on miR-27 knockdown and normalized phenotype of the double morphants.
miR-27b promotes angiogenesis and branching by blocking Spry2
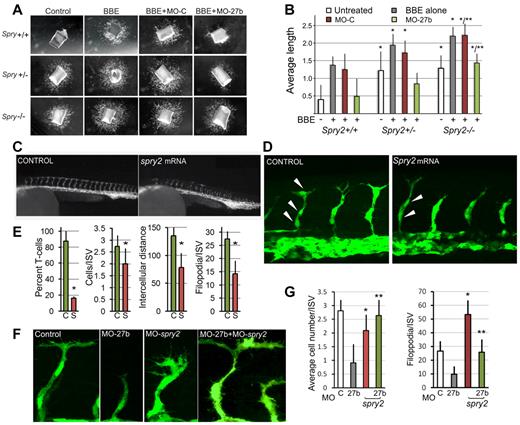
Spry2 inhibits secondary branching in developing vertebrate lung7 and auditory sensory epithelium.27 In angiogenesis, simultaneous suppression of Spry2 and Spry4 accelerated reperfusion in a mouse model of limb ischemia10 and dominant-negative Spry2 caused vascular regression in the healing skin wounds.33 Therefore, it is feasible that miR-27b exerts its effects through suppression of Spry2. The wild-type, heterozygous Spry2+/− or null Spry2−/− aortic rings all mounted vigorous angiogenesis in response to BBE (Figure 4A-B) and this response was increased when at least one Spry2 allele was missing. Moreover, aortic rings from the Spry2+/− and Spry−/− mice showed robust neo-angiogenesis even in the absence of pro-angiogenic BBE, whereas the aortas from their wild-type littermates displayed no or little sprouting (Figure 4A-B). Therefore, the loss of a single Spry2 allele was sufficient to release angiogenesis. In agreement, MO-27b inhibited sprouting of the wild-type, Spry2+/+ aortas more effectively than in heterozygous Spry+/− aortic rings and had negligible effect on Spry2-null aortas, indicating that blocking Spry2 is a critical component of angiogenesis by miR-27b (Figure 4A-B).
Spry2 represses vascular branching. (A) Aortic rings from 3- to 5-week-old mice (Spry+/− and Spry−/−, respectively) and from age-matched wild-type control littermates (Spry+/+) were embedded in Matrigel and treated with control medium (Control) and pro-angiogenic BBE (200 μg/mL) with or without miR-27b targeting or control MOs (MO-27b and MO-C). Vascular sprouting was documented after 4 days. (B) The average sprout length was measured in 6-8 samples with ImageJ software. *P < .0035 for the difference from noninduced wild-type controls and **P < .0002 for the difference from BBE-stimulated wild-type controls. (C) Fertilized zebrafish eggs were injected with mRNA encoding Spry2 (200 ng/injection) or control mRNA and the embryos were photographed at 36 hpf. Note the lack of T-shaped cells at the tips of the ISVs. (D) Confocal images of the embryos overexpressing Spry2 and control (C). Note the lack of T-shaped tip cells and decreased filopodia formation. (E) Quantification of the experiment in panel D. The percentage of vessels with T-shaped tip cells at the end was determined in the trunk area of 5 representative embryos. *P < 5 × 10−7, P < .0007, P < .009, and P < .0002, respectively, for the differences from the controls. (F) Zebrafish embryos were injected with MO-27b, MO-Spry2, or both. Confocal images of the vasculature are shown. Note premature branching and increased filopodia formation in the Spry2 morphants and normalized phenotype of the double morphants. (G) Quantitative analysis of the experiment in panel F. *P < .00086 for the difference from controls; **P < .002 for the difference from MO-27b alone.
Spry2 represses vascular branching. (A) Aortic rings from 3- to 5-week-old mice (Spry+/− and Spry−/−, respectively) and from age-matched wild-type control littermates (Spry+/+) were embedded in Matrigel and treated with control medium (Control) and pro-angiogenic BBE (200 μg/mL) with or without miR-27b targeting or control MOs (MO-27b and MO-C). Vascular sprouting was documented after 4 days. (B) The average sprout length was measured in 6-8 samples with ImageJ software. *P < .0035 for the difference from noninduced wild-type controls and **P < .0002 for the difference from BBE-stimulated wild-type controls. (C) Fertilized zebrafish eggs were injected with mRNA encoding Spry2 (200 ng/injection) or control mRNA and the embryos were photographed at 36 hpf. Note the lack of T-shaped cells at the tips of the ISVs. (D) Confocal images of the embryos overexpressing Spry2 and control (C). Note the lack of T-shaped tip cells and decreased filopodia formation. (E) Quantification of the experiment in panel D. The percentage of vessels with T-shaped tip cells at the end was determined in the trunk area of 5 representative embryos. *P < 5 × 10−7, P < .0007, P < .009, and P < .0002, respectively, for the differences from the controls. (F) Zebrafish embryos were injected with MO-27b, MO-Spry2, or both. Confocal images of the vasculature are shown. Note premature branching and increased filopodia formation in the Spry2 morphants and normalized phenotype of the double morphants. (G) Quantitative analysis of the experiment in panel F. *P < .00086 for the difference from controls; **P < .002 for the difference from MO-27b alone.
In contrast, Spry2 overexpression in zebrafish embryos (microinjections of Spry2 mRNA into fertilized eggs) resulted in a failure to form DLAVs at 24 and 36 hpf (Figure 4C), likely because of the lack of T-shaped (tip) cells. At 24 hpf, in the embryos overexpressing Spry2, less than 20% of the ISVs had typical T-shaped cells, compared with approximately 90% in control embryos (Figure 4C-E). Moreover, Spry2 overexpression reduced the average cell number per ISV to approximately 2, compared with the normal 3 in controls (Figure 4D-E). Excess Spry2 reduced the distances between the nuclei and diminished the number of filopodia per ISV (Figure 4D-E).
Blocking Spry2 with targeting MO (MO-Spry2) was sufficient to rescue the overall lack of vascular sprouting in the miR-27b morphants at 24 hpf (Figure 4F). In contrast to Spry2 overexpression, Spry2 morphants showed a clear tendency for premature ISV branching after reaching 2 cells in length (instead of the typical 3) and increased filopodia number (Figure 4F-G). Coinjection of MO-Spry2 with MO-27b restored the normal phenotype of the miR-27b morphants (Figure 4F and supplemental Figure 5). The endothelial cell number per ISV was restored to the average 3 (Figure 4G) and the filopodia number per vascular sprout, which reflects tip cell fate and was significantly decreased by MO-27b, returned to near normal levels (Figure 4G).
miR-27b regulates arterial-venous segregation
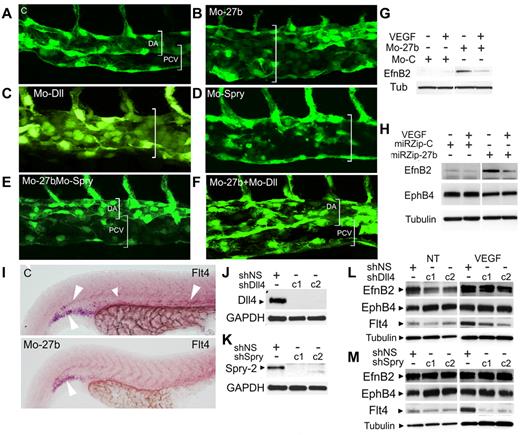
In zebrafish, endothelial cells in the precursor DA selectively assume venous fate, sprout, branch and form a precursor posterior cardinal vein (PCV).34 This process is tightly regulated, and requires VEGF-dependent expression of Dll4, EphB2, and its receptor, EphB4. In control embryos, lumenized PCV precursor was formed at 32 hpf (Figure 5A). In contrast, miR-27b morphants at 32 hpf formed only one dorsal vessel (Figure 5B). Consistent with previous observations,34 Dll4 knockdown also caused the formation of a single dorsal vessel (Figure 5C). Surprisingly, Spry2 knockdown also resulted in the lack of segregation (Figure 5D), suggesting the importance of Spry2 for PCV formation. In miR-27b morphants, additional knockdown of either Dll4 or Spry2 was sufficient to restore, at least in part, normal PCV (Figure 5E-F), supporting their critical roles in primary vein morphogenesis. Using rPEDF or by blocking miR-27b with MO-27b or lentiviral miRZip-27b, we up-regulated EphB2 and down-regulated EphB4 in cultured endothelial cells (Figure 5G-H and supplemental Figure 6), suggesting that miR-27b promotes venous specification. In agreement, the expression of flt4 (vegfr3), which is necessary for arterial morphogenesis in zebrafish,25 was visibly reduced in miR-27b morphants compared with controls (Figure 5I). As expected, Dll4 knockdown in cultured endothelial cells (Figure 5J) reduced the arterial marker efnB2 and increased the venous marker ephB4 (Figure 5L). Spry2 knockdown (Figure 5K) had no effect on efnB2, but increased ephB4 (Figure 5M). Finally, Flt4 was down-regulated when Spry2 was silenced (Figure 5L-M and supplemental Figure 7).
miR-27b regulates arterial-venous segregation. (A-F) miR-27b, Dll4, and Spry2 were silenced and arterial-venous segregation observed at 32 hours. (A) Segregation of the DA and PCV in control morphants. (B-D) Defective PCV morphogenesis in the embryos injected with MO-27b (B), MO-Dll (C), and MO-Spry2 (D). (E-F) Rescue of PCV morphogenesis in double morphants. (G-H) miR-27b inhibitors increase arterial phenotype. miR-27b was blocked with MO-27b (G) or miR-Zip lentiviral vectors (H) and EphB2 and EphB4 were measured by Western blot. (I) miR-27b regulates Flt4. In situ hybridization for the whole-mount zebrafish embryo. (J-M) Dll4 and Spry2 maintain arterial phenotype. Dll4 and Spry2 were silenced in cultured microvascular endothelial cells using lentivirally expressed shRNA (J-K). Distinct shRNA sequences are indicated as c1 and c2. (L-M) Endothelial cells infected with control vector, Dll4, or Spry shRNA were left untreated or treated with 1 ng/mL of VEGF, and the expression of EphB2, EphB4, and Flt4 was measured by Western blot.
miR-27b regulates arterial-venous segregation. (A-F) miR-27b, Dll4, and Spry2 were silenced and arterial-venous segregation observed at 32 hours. (A) Segregation of the DA and PCV in control morphants. (B-D) Defective PCV morphogenesis in the embryos injected with MO-27b (B), MO-Dll (C), and MO-Spry2 (D). (E-F) Rescue of PCV morphogenesis in double morphants. (G-H) miR-27b inhibitors increase arterial phenotype. miR-27b was blocked with MO-27b (G) or miR-Zip lentiviral vectors (H) and EphB2 and EphB4 were measured by Western blot. (I) miR-27b regulates Flt4. In situ hybridization for the whole-mount zebrafish embryo. (J-M) Dll4 and Spry2 maintain arterial phenotype. Dll4 and Spry2 were silenced in cultured microvascular endothelial cells using lentivirally expressed shRNA (J-K). Distinct shRNA sequences are indicated as c1 and c2. (L-M) Endothelial cells infected with control vector, Dll4, or Spry shRNA were left untreated or treated with 1 ng/mL of VEGF, and the expression of EphB2, EphB4, and Flt4 was measured by Western blot.
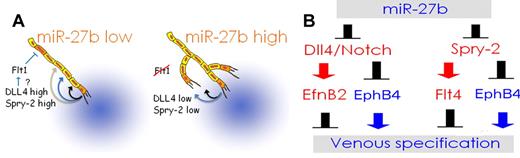
In conclusion, the results of the present study show that angio-inhibitory factors can control angiogenic balance by blocking miR-27b. In the absence of miR-27b, Dll4 and Spry2 are expressed at high levels and repress tip cell fate, vascular sprouting, and branching (Figure 6). In developing embryos, miR-27b maintains proper arterial-venous segregation, whereas Dll4/Notch and Spry2 oppose venous specification (Figure 6).
Schematic representation of miR-27b functions. (A) Regulation of vascular sprouting. At low levels, miR-27b allows the expression of Spry2 and Dll4, which block capillary branching and tip endothelial cell fate, respectively. In addition, miR-27 down-regulates Flt1, possibly via Dll4/Notch. Flt1 also represses tip endothelial cell fate and therefore vascular sprouting. When miR-27b levels are high, Spry2, Dll4, and Flt1 are low and allow for the increased occurrence of the tip cells and therefore denser capillaries. (B) Regulation of venous specification. In endothelial cells of the early DA, miR-27b blocks Dll4 expression. Consequently, Notch signaling weakens, causing lower EfnB2 expression, whereas the venous marker EphB4 increases. At the same time, the repression of Spry2 releases EphB4 expression and lowers Flt4. The net result is a sum of increased venous markers, decreased expression of arterial genes, and accelerated venous differentiation.
Schematic representation of miR-27b functions. (A) Regulation of vascular sprouting. At low levels, miR-27b allows the expression of Spry2 and Dll4, which block capillary branching and tip endothelial cell fate, respectively. In addition, miR-27 down-regulates Flt1, possibly via Dll4/Notch. Flt1 also represses tip endothelial cell fate and therefore vascular sprouting. When miR-27b levels are high, Spry2, Dll4, and Flt1 are low and allow for the increased occurrence of the tip cells and therefore denser capillaries. (B) Regulation of venous specification. In endothelial cells of the early DA, miR-27b blocks Dll4 expression. Consequently, Notch signaling weakens, causing lower EfnB2 expression, whereas the venous marker EphB4 increases. At the same time, the repression of Spry2 releases EphB4 expression and lowers Flt4. The net result is a sum of increased venous markers, decreased expression of arterial genes, and accelerated venous differentiation.
Discussion
miR-27b maps to the miR-23b cluster localized on mouse chromosome 13 (human chromosome 9). To date, miR-27b has been ascribed functional roles in adipocytes35 and skeletal muscle.36 Early screens point to miR-27b,16 but elucidate neither its cellular functions nor its molecular targets. A more recent study demonstrates a pro-angiogenic function of the miR-23 cluster, which includes miR-27b, and identifies its molecular targets as Spry2 and Semaphorin.28 Our study is the first to demonstrate the critical role of miR-27b in the control of the Notch pathway, regulation of tip cell fate, vascular branching, and venous specification in vascular endothelium. In addition, we have clearly shown the role of Spry2 in the control of vascular sprouting and arterial specification, functions that have not been recognized previously.
Our study reveals a critical role of miR-27b in vascular patterning and positions it at the crux of angiogenic balance, where it is up-regulated by proangiogenic factors and down-regulated by anti-angiogenic proteins. Therefore, miR27b may serve as a master coordinator of pro- and antiangiogenic signals at the posttranscriptional level, adding another layer of complexity to the regulation of angiogenesis. Second, we discovered that miR-27b promotes microvessel sprouting and branching by repressing 2 key proteins, Dll4 and Spry2. Dll4 is the major inhibitor of the tip cell fate32 and Spry2 is a key repressor of branching morphology.7 Previous studies showed that ectopic Dll4 knockdown increases cellular content and the number of endothelial tip cells in zebrafish intersegmental arteries24 and augments filopodia formation and vascular density in the mouse retina.37 Another Notch ligand, Jag1, causes the opposite effects and increases the number of tip cells and endothelial sprouts by competing with Dll4 for Notch binding.38 In the present study, we show that miR-27b blocks Dll4/Notch interactions and therefore increases the number of the endothelial cells committed to tip fate and, subsequently, augments capillary density.
The role of Spry2 and Spry4 in angiogenesis and Spry2 regulation by the miR-23/27 cluster has been demonstrated previously.28,39 The control of Spry1 and Spry2 has been previously attributed to miR-21,40,41 and its regulation by miR-27b has not been demonstrated. On a molecular level, Spry2 inhibits signaling by MAP kinases and Rho GTPases.8,42,43 Repressed Rho GTPase signaling and impaired actin polymerization are consistent with diminished distance between cell nuclei and poor filopodia formation in the ISV of zebrafish injected with Spry2 mRNA. However, blocking proliferation and migration were thought to be its main functions in the vascular endothelium.33,43 In the present study, we demonstrate that in zebrafish, Spry2 blocks the emergence of T-shaped ISV tip cells. Moreover, Spry2 clearly regulates the key genes involved in arterial-venous specification, a completely novel function. Spry2 knockdown moderately increased the venous marker EphB4 and reduced Flt-4; therefore, like Notch, Spry2 promotes venous differentiation.
We have also showed that in the developing zebrafish embryo, blocking either Dll4 or Spry2 rescued defective vascular sprouting caused by miR-27b knockdown. Simultaneous control by miR-27b of the 2 major pathways that regulate branching and sprouting is an economical way to manage vascular patterning. The fact that miR-27b, via its primary targets, also controls the expression of flt4 suggests that it may promote the vascular versus lymphatic endothelial fate.44 Interestingly, miR-27b suppresses Flt-1, which is known to block tip cell fate via the Notch pathway.45 Further studies are needed to determine whether Spry2 is involved in the regulation of Flt-1 by miR-27b.
Dll4 and Notch are intimately linked with arterial-venous specification.24,46 Dll4 activates Notch and its target genes (mammalian Hey1/2 and zebrafish gridlock), which promote arterial differentiation. Mice null for Dll4 fail to maintain arterial identity. Conversely, the orphan nuclear receptor COUP-TFII determines venous phenotype by repressing Notch and promoting the expression of EphB4 and VEGFR3/Flt4.47 Recently, a novel type of arterial-venous segregation has been identified in zebrafish, whereby select endothelial cells in the DA assume venous fate and form downward sprouts, which subsequently bifurcate, fuse, lumenize, and form the PCV.34 This process requires Dll4 to restrict the number of sprouting precursors and to maintain the arterial fate of the DA; the blockade of Notch signaling with DAPT or Dll4 MOs causes excessive sprouting and the formation of a single large vessel. Interestingly, miR-27b silencing also led to formation of a single vessel in place of DA and PCV, which was enlarged likely because of continuous proliferation. In addition, miR-27b knockdown increased the level of arterial marker EphB2. When sprouting was restored in miR-27b morphants by silencing Dll4 or Spry2, arterial-venous segregation was rescued. Moreover, miR-27b silencing increased endothelial EphB2 and decreased EphB4, suggesting that miR-27b drives venous specification and subsequent segregation of the endothelial cells to form the precursor vein. Interestingly, Spry2 is known to block phosphorylation of EphB1 and subsequent segregation of the cells expressing EphB2 ligand from those expressing EphB1 receptor.43 Furthermore, in our study, Spry2 knockdown significantly reduced the expression of Flt4 (VEGFR3), the interaction of which with VEGFR2 is required for establishing arterial identity.25
miR-27b is the second miRNA after miR-126, which is necessary for the development and maintenance of embryonic vasculature.20,21 The role of miR-27b in tumor vascularization is not entirely clear. Despite normalization (increased circumference) of the tumor vasculature, its effect on tumor size was only modest. Conversely, MO-27b visibly reduced proliferation of tumor cells, suggesting that miR-27b may have tumor-promoting effects independent of angiogenesis. Interestingly, the miR-27b and miR-126 targets Spry2 and SPRED1, respectively, both belong to the Sprouty superfamily and are both likely to repress branching of the tubular structures. Therefore, it is not unlikely that miR-126 regulates vascular sprouting. In agreement with this, retinal vasculature of miR-126 null mice shows a decreased number of sprouts and filopodia at the vascular front.48
miR-27b integrates 2 opposing vascular processes, expansion and regression, which are controlled by pro- and antiangiogenic factors, respectively. In the present study, we show that miR-27b is blocked by angiogenesis inhibitors and acts as a critical component of the angiogenic switch. These results suggest miR-27b as a candidate therapy target for cancer and heart disease.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank E. Olson, N. Lawson, and A. Aurora for helpful discussions, and Dr S. Getsios for the gift of Abs.
This work was funded by the National Institutes of Health (grants RO1 HL068033 to O.V., R21CA139311 to S.R., R21CA139311 to J.L., and T32 DK062716 to D.V.).
National Institutes of Health
Authorship
Contribution: D.B. and D.V. designed and performed the experiments, analyzed the data, and prepared the figures; J.T. and J.M.T. provided critical reagents, designed the experiments, analyzed the data, and participated in writing the manuscript; I.M., E.V., and A.L.R. performed the experiments; J.D.L. provided critical reagents and contributed to discussions about the data; S.Y.R. provided critical reagents and participated in designing the experiments and writing the manuscript; and O.V.V. designed and supervised the experiments, analyzed the data, prepared the figures, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olga V. Volpert, PhD, Urology Department, Northwestern University Feinberg School of Medicine, 303 E Chicago Ave, Chicago, IL 60611; e-mail: olgavolp@northwestern.edu.
References
Author notes
D.B. and D.V. contributed equally to this work.

![Figure 3. miR-27b controls tip cell fate via Dll4. (A) Representative images of zebrafish vasculature in miR-27b, Dll4, and double miR-27b/Dll4 morphants. Arrowheads indicate disconnected, hyper-migratory tip cells (MO-Dll4 [1]) and excessive branching (MO-Dll4 [2]) in Dll4 morphants. Note decreased sprout length, lack of branched tip cells, and decreased filopodia formation in miR-27b morphants and restored phenotype in double morphants. Broken line indicates that the image is a composite of 2 independent photomicrographs of the same field taken in different focal planes (B-C). Quantitative analysis of experiment shown in panel A. *P < .0002 and **P < .00007 between control and miR-27b knockdown. (D) Expression of flt1 in miR-27b, Dll4, and double morphants. Note increased Flt1 mRNA on miR-27 knockdown and normalized phenotype of the double morphants.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/11/10.1182_blood-2011-07-370635/4/m_zh89991286230003.jpeg?Expires=1769091829&Signature=c3iSLpfdBJD2VNVuP2r7F5PZkeXny5-dGQfLbvnYXPEPyVs-KsAQfKOYEea8VFT7Q~nghEQoBUwxkxAm1pD2ezNJRS5xaG8MhgUp4oJ4kHWPEin6y4~cIlLl1PvSS1YRL6iyt5AlyENF8lq3dBsY4tMUiO0aeJFwYqvw~Li3h-WOonQExo2EK-MxEBmSksaDXTV9Lf2QxVpmX7R5kcncDUweyKeYPJpFH0O7yxW-5o2ihP2cjsURRLFbKAzONmObwx-Vyb9uRWm4-AosKzZI~FOgVGATfXkzQiMDlKDxfkzXD0JywIWfVIu4TqVZk4j4O78-RUc9vy8iR63zbPdlDQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)