Abstract
Antigen-activated T lymphocytes undergo an immune or tolerogeneic response in part according to the activation status of their antigen-presenting cells. However, factors controlling the activation of antigen-presenting cells are not fully understood. In this study, we demonstrate that immune tolerance after organ allotransplantation in the rat is associated with a repressed intragraft expression of several enzymes of the trans-sulfuration pathway, including cystathionine γ-lyase (CSE). The pharmacologic blockade of CSE with propargylglycine delayed heart allograft rejection and abrogated type IV hypersensitivity but did not modify antibody responses, and was associated with a selective inhibition of the TH-1 type factors T-bet, IL-12, and IFN-γ. IL-12 repression could also be induced by propargylglycine in vitro in monocytes and dendritic cells (DCs), a phenomenon not mediated by changes to nuclear factor-κ B or hydrogen sulfide but that occurred together with a modulation of intracellular cysteine content. Intracellular cysteine levels were predominantly controlled in DCs by CSE activity, together with extracellular import via the Xc− transporter. Our results indicate that CSE plays a critical role in regulating IL-12 in monocytes and DCs and is down-modulated in transplant tolerance, presumably participating in the maintenance of the tolerant state.
Introduction
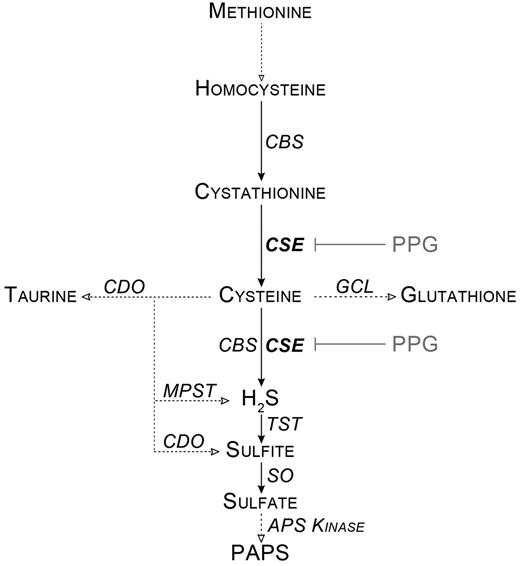
Cystathionine γ-lyase (CSE) is a key pyridoxal-phosphate-dependent enzyme in the trans-sulfuration pathway. Trans-sulfuration links methionine metabolism to the biosynthesis of cellular redox-controlling molecules, such as cysteine, glutathione, and taurine. CSE also controls the metabolism of cysteine into hydrogen sulfide (H2S), pyruvate, and ammonium ions (Figure 1). CSE is expressed in peripheral tissues, whereas in the central nervous system, the trans-sulfuration pathway and H2S production are driven by the cystathionine-β-synthase enzyme.1
Simplified scheme of the cysteine metabolism. Dotted lines with empty arrows represent multistep conversions. APS Kinase indicates adenosine 5′-phosphosulfate kinase; CBS, cystathionine-β-synthase; CDO, cysteine dioxygenase; GCL, glutamate cysteine ligase; MPST, mercaptopyruvate sulfurtransferase; PAPS, 3′-phosphoadenosine 5′-phosphosulfate; SO, sulfite oxidase; and TST, thiosulfate sulfurtransferase.
Simplified scheme of the cysteine metabolism. Dotted lines with empty arrows represent multistep conversions. APS Kinase indicates adenosine 5′-phosphosulfate kinase; CBS, cystathionine-β-synthase; CDO, cysteine dioxygenase; GCL, glutamate cysteine ligase; MPST, mercaptopyruvate sulfurtransferase; PAPS, 3′-phosphoadenosine 5′-phosphosulfate; SO, sulfite oxidase; and TST, thiosulfate sulfurtransferase.
T lymphocytes, being deficient in CSE and inefficient at importing cystine (the predominant form of this amino acid in the extracellular milieu), are metabolically dependent on dendritic cells (DCs) for cysteine import and glutathione (GSH) synthesis,2 2 molecules with major antioxidant properties required for T-cell activation and proliferation.3,4 A control on cysteine and GSH availability is part of the mechanism of action of regulatory T cells (Tregs) and myeloid-derived suppressor cells to mediate immune suppression. Tregs do so by limiting expression of γ-glutamate cysteine ligase in DCs, the limiting enzyme for GSH synthesis,5,6 and myeloid-derived suppressor cells by limiting DC-induced cysteine feeding of effector T cells.7 GSH is thought to regulate IL-12 production in DCs and macrophages, probably through the GSH/GSSG balance (the oxidized form of GSH) that determines the intracellular redox state and regulates p38 MAPK and calmodulin expression, which in turn binds and sequesters c-rel in the cytoplasm.8 In addition, GSH depletion reduces the expression of costimulatory molecules in DCs and modifies their ability to stimulate allogeneic Mixed leukocyte reaction (MLR) responses of T cells.9 In vivo, GSH depletion in antigen-pulsed DCs interferes with the delayed-type hypersensitivity (DTH) response, probably as a consequence of IL-12 repression.10
H2S is the final metabolite of the trans-sulfuration pathway. It is now recognized as the third endogenously produced gasotransmitter after nitric oxide (NO) and carbon monoxide (CO), which induce important physiologic modifications in mammals, such as vasomodulation, synaptic sensitivity, inflammation, and the modulation of immune responses.11 Among gasotransmitters, the role of H2S in immune responses is the least understood. It has been deduced from CSE blockade experiments that H2S was involved in protecting mice from lipopolysaccharide (LPS)–induced endotoxemia,12 pancreatitis,13 and sepsis.14 However, other studies have shown that inhibition of H2S production increased leukocyte-endothelium adherence as well as carrageenan-induced leukocyte infiltration and paw edema, whereas H2S donors inhibited those effects.15,16 Thus, pro-inflammatory as well as anti-inflammatory roles have been assigned to H2S.
Immune tolerance to transplanted allografts refers to as an adaptation of the recipient's immune system that either fails to recognize donor alloantigens or controls alloreactivity. It is controlled by the central, thymic depletion of alloreactive T cells and/or by peripheral regulatory cells.17 In experimental transplantation, several T-cell subsets with suppressive activity have been described and among them predominate the CD4+CD25+forkhead box P3 (FoxP3)+ Tregs. It is becoming increasingly clear that cell types other than T lymphocytes cooperate and form a network of cellular interactions controlling immune responses. These cells include tolerogenic DCs, alternatively activated macrophages, mesenchymal stem cells, B cells, and myeloid-derived suppressor cells. Tolerogenic DCs are in an immature state and present antigens in a subimmunogenic manner, in the absence of costimulatory signals, and in a context where proinflammatory cytokine production is inhibited. Several other mechanisms may be responsible for the peripheral tolerance induced by immature DCs, among them the production of IL-10 and TGF-β.18
In this study, we linked experimental transplant tolerance to the down-regulation of intragraft CSE and found a role for this enzyme in the regulation of DC function. Our results highlight the important role of the trans-sulfuration pathway led by CSE in directly controlling intracellular cysteine levels and IL-12 synthesis by DCs, independently of H2S. In vivo, the activity of CSE might also regulate IL-12 levels and specifically control type-1 immune responses and transplant tolerance.
Methods
Chemicals and reagents
Ethyl chloroformate, ethyl acetate (99.8% purity or better), NH4OH, sulfosalicylic acid, Dowex 50 W-X8 resin, L-cysteine, D,L-propargylglycine, sulfasalazine, β-cyano-alanine, NaHS, and keyhole limpet hemocyanin (KLH) were purchased from Sigma-Aldrich. L-[3,3-2H2]Cysteine (98 atom%) was obtained from Cambridge Isotopes. All other reagents used for gas chromatography-mass spectrometry (GC-MS) analyses were of analytical grade. GYY 4137 was purchased from Cayman Chemicals. Unless specified, all other reagents were from Sigma-Aldrich.
Organ transplantation, tolerance induction, and treatments
Lewis 1W (LEW.1W, haplotype RT1u) and Lewis 1A (LEW.1A, haplotype RT1a) rats (Center d'Elevage Janvier) share the same genetic background but are MHC I and II mismatched. Heterotopic transplants of 8- to 12-week-old LEW.1W hearts into LEW.1A recipients were carried out according to the technique described by Ono and Lindsey.19 For syngeneic transplants, LEW.1A hearts were grafted on LEW.1A recipients. Live-sustaining allografts of LEW.1W kidneys in LEW.1A rats were performed aseptically, and a binephrectomy was performed 7 days after transplantation as previously described.20 Blood urea and creatinine and urine protein/creatinine ratios were measured throughout the posttransplantation period. Blood urea less than 8mM and blood creatinine less than 40mM were considered normal.21 Transplant tolerance induction was obtained by treating rats with one of the following regimens (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article): anti-CD28 antibodies,22 antidonor anti-class II antibodies,21 donor-specific blood transfusions,23 CD40-Ig,24 or LF015-095, a deoxyspergualin analog.25 De novo heart-transplanted rats were injected intraperitoneally for 20 days with propargylglycine (PPG; 50 mg/kg per day), an irreversible CSE inhibitor, supplemented with Nacystelyn, a lysine salt of N-acetylcysteine (NAL; 500 mg/kg per day) given to avoid neuronal toxicity. Heart graft survival was evaluated by daily palpation through the abdominal wall. Rats were maintained in an specific pathogen-free animal facility according to the institutional guidelines of Inserm.
MLRs
MLRs were performed as previously described.26 Briefly, T cells from Sprague-Dawley rats (8-12 weeks old) or transplanted rats were purified and depleted from His24 (CD45R), OX42 (CD11b/c), and 3.2.3 (CD161) positive cells with magnetic beads (Dynal). Thirty-five gray-irradiated antigen-presenting cells (APCs; 104 cells or 2 × 104 cells) were enriched from transplanted rat spleens by a 14.5% Nicodenz (Gentaur) gradient. After 2 days, supernatants were harvested and stored at −20°C for cytokine content determination. Alternatively, after 5 days, proliferation was measured by 3H-thymidine (GE Healthcare; 1 μCi/well) uptake during the last 16 hours of culture.
Immunostaining and flow cytometry
Graft samples were embedded in Tissue Tek OCT compound, snap-frozen in liquid nitrogen, cut into 5-μm sections, and fixed in acetone. Tissue analysis was performed after saturation using PBS, 4% BSA, 10% goat serum. Immunohistologic analysis of the infiltration was evaluated using a mixture of CD45 and TCRα/β mAbs (OX1 + OX30 and R7.3), which were produced in our laboratory from hybridoma obtained from the ECACC. After washing, cells were incubated for one hour at room temperature with FITC-conjugated anti–mouse IgG diluted 1/100 (Jackson ImmunoResearch Laboratories) and mounted with Dako medium (Dako North America) and analyzed on a Zeiss Axioskopz microscope. Images were acquired with a Zeiss AxioCamHRC camera and processed using Zeiss AxioVisionRel, Version 4.2 software. Control sections were performed by replacing the primary Abs with dilution buffer. Cell suspensions were labeled with anti-TCRα/β peridinin chlorophyll protein-conjugated Ab, FITC-conjugated anti-CD4 Ab, and allophycocyanin-conjugated anti-FoxP3 Ab or were stained with PE-conjugated anti-CD45R (His24) Ab, Alexa488-conjugated anti-CD11b (Ox42) Ab, peridinin chlorophyll protein-conjugated anti–Class II (Ox6) Ab, and Alexa647-conjugated anti-CD103 (Ox62) Ab, all from BD Biosciences.
RNA preparation, microarray analysis, and real-time quantitative PCR
Total RNA obtained by Trizol extraction was cleaned up using RNeasy columns (QIAGEN). RNA quantity and quality were determined using a NanoDrop spectrophotometer and an Agilent 2100 bioanalyzer. The Applied Biosystems rat genome survey microarray (part no. 4337467) contained 26 857 60-mer oligonucleotide probes representing 27 088 individual rat genes. Digoxigenin-UTP labeled cRNA was generated and amplified from 0.5 μg of total RNA from each sample using an Applied Biosystems NanoAmp chemiluminescent reverse transcriptase-in vitro transcription labeling kit (part no. 4365715). Array hybridization was performed for 16 hours at 55°C. Chemiluminescence detection, image acquisition, and analysis were performed using the Applied Biosystems chemiluminescence detection kit (part no. 436875D), analyzer (part no. 4338036), and Version 1.1 analyzer software (part no. 4336391) according to the manufacturer's protocol. Microarray raw data were analyzed by the R language and environment for statistical computing and graphics. Genes were identified using the Panther Protein Classification System Probe ID database. Microarray data corresponding to syngeneic kidney transplantation in the rat and to kidney allotransplantation induced by antidonor class II antibodies21 were deposited onto the Gene Expression Omnibus web-based data repository (reference no. GSE28474). Real-time quantitative PCR was performed in an Applied Biosystems GenAmp 7700 sequence detection system using SYBR-Green PCR core reagents as previously described21 or TaqMan gene expression assays according to the manufacturer's instructions (Applied Biosystems).
TaqMan probes (Applied Biosystems) were used for CSE (Rn00567128-m1), T-Bet (Rn01461633-m1), GATA-3 (Rn00484683-m1), IL-1β (Rn01514151-m1), IL-4 (Rn01456866), and HPRT (Rn01527840-m1) Otherwise, the following primers were used in this study:
HPRT, CCTTGGTCAAGCAGTACAGCC (forward) and TTCGCTGATGACACAAACATGA (reverse); IFN-γ, TGGATGCTATGGAAGGAAAGA (forward) and GATTCTGGTGACAGCTGGTG (reverse); IL-12p35, TGATGATGACCCTGTGCCTT (forward) and GCATGGAGCAGGATACAGAGC (reverse); IL-12p40, TCATCAGGGGACATCATCAAACC (forward) and CGAGGACGCACCTTTCTG (reverse); IL13, TATGGAGCGTGGACCTGACA (forward) and GCGGAAAAGTTGCTTGGAGTA (reverse); iNOS, GACCAAACTGTGTGCCTGGA (forward) and TACTCTGAGGGCTGACACAAGG (reverse); HO-1, CCACAGCTCGACAGCATGTC (forward) and GTTTCGCTCTATCTCCTCTTCCA (reverse); TGFβ1, CTCAACACCTGCACAGCTCC (forward) and ACGATCATGTTGGACAACTGCT (reverse); LAG-3, ATATGAATTCACAGAGGAGATGAGGCAG (forward) and ATATGAATTCTCCTGGTCAGAGCTGCCT (reverse); IDO, GCTGCCTCCCATTCTGTCTT (forward) and TGCGATTTCCACCATTAGAGAG (reverse); IL-10, CTTCACCTGCTCCACTGCC (forward) and CCTGGTAGAAGTGATGCCCC (reverse); IL-6, GCAAGAGACTTCCAGCCAGTT (forward) and CATCATCGCTGTTCATACAATCA (reverse); IL-17A, TGCTGTTGCTGCTACTGAACC (forward) and AACTTCCCCTCAGCGTTGAC (reverse); IL-23p19, GGACTCGGACATCTTCACAGG (forward) and GGAACGGAGAAGAGAACGCT (reverse); ROR-γT, GCAGGAGCAATGGAAGTCG (forward) and CGCTGAGGAAGTGGGAAAA (reverse); and FoxP3, CTGCGTATGTCCTTCCCTCC (forward) and AACCTTAGTGCCTGATGTGCC (reverse).
HPRT was used as an endogenous control gene to normalize the various starting amounts of RNA. Relative expression between a given sample and a control sample was calculated with the 2−ΔΔCt threshold cycle method (ABI PRISM 7900 user bulletin 2:11-24, 1997; PE Applied Biosystems). All samples were analyzed in duplicate. Data are available on the Gene Expression Omnibus web-based data repository (accession no. GSE28474).
CSE enzymatic activity measurement
H2S-synthesizing activity was determined as previously described.27 Briefly, heart transplants were homogenized (Ultra-Turrax) in 100mM ice-cold potassium phosphate buffer (pH 7.4). The reaction mixture (1 mL) contained L-cysteine (10mM; 40 μL), pyridoxal 5′-phosphate (2mM; 40 μL), and tissue homogenate (920 μL; 7-12 mg of protein, using the BCA method). A total of 0.5 mL of 1% zinc acetate was added in the central well for trapping evolved H2S from the mixture. After flushing the flasks with N2, the catalytic reaction was initiated by transferring the flask from ice to a 37°C shaking incubator. After 120 minutes, the reaction was stopped by adding 0.5 mL of 50% trichloroacetic acid, and the flasks were left an additional 60 minutes to complete trapping of H2S. The content of the central well was transferred to an Eppendorf tube and mixed with 200 μL of 72mM N,N-dimethyl-p-phenylenediamine dihydrochloride in 7.1M H2SO4 and left for 10 minutes to react. To each tube, 50 μL of 185mM FeCl3 in 15mM H2SO4 was added and left for another 10 minutes. After this, the optical absorbance of the resulting solution at 670 nm was measured on a spectrophotometer. The H2S concentration in the solution was calculated against a calibration curve of NaHS. The H2S production is expressed as nanomoles H2S formed per milligram of protein per hour.
Human MoDC generation and cell maturation
Human peripheral blood monocytes (> 90% pure) were obtained from the Plateforme DTC (IFR26) by countercurrent elutriation. Monocytes were differentiated to immature DCs by culture (2 × 106 cells/mL) for 5 days in RPMI 1640 medium with 10% FCS, 2mM glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin supplemented with IL-4 (400 IU/mL; CellGro), and GM-CSF (1000 IU/mL; Gentaur). For their maturation, immature DCs or THP-1 cells were cultured for 24 hours with LPS in the presence of excipient, PPG, BCA, GYY4137, NaHS, or sulfasalzine (SAS).
MoDC transfections with siRNA
Monocyte-derived DCs (MoDCs) were transfected in 6-well plates at day 4 after differentiation, using RNAi Max lipofectamine (Invitrogen) and siRNA targeting CSE (Ambion, AM16708, 61% knockdown) or a scrambled negative control (Ambion, AM4615) at a final concentration of 32.5nM. High transfection efficiency (> 85%) was verified with fluorescent siRNA (Fluorescent Block-IT, Invitrogen). Maturation of transfected DCs was performed 24 hours after transfection using the same protocol as described in “Human MoDC generation and cell maturation.”
Assessment of NFκB p65 activation
MoDCs were matured with LPS and treated with PPG for 1 or 2 hours. NF-κB DNA binding activity was assessed using the TransAM NF-κB family TF assay kit (Active Motif). Nuclear fractions were harvested from cells using the nuclear extraction kit according to the manufacturer's instructions. The protein concentration was determined using the BCA method (Pierce). A total of 5 μg of nuclear extract in cell binding buffer was added in triplicate wells containing the 5′-GGGACTTTCC-3′ NF-κB consensus binding site oliogonucleotide.
Sample preparation of human monocytes, MoDCs, and THP-1 lysates for cysteine content determination by GC-MS
Cellular maturation was carried out as described in “Human MoDC generation and cell maturation.” Cells were resuspended in PBS with 10 nmol of L-[3.3-2H2]cysteine added as an internal standard, and sonicated on ice. After deproteinization, cysteine was purified by cation exchange chromatography using NH4OH 10M for elution. After this, samples and standard solutions were dissolved in 0.2mM sodium phosphate buffer (pH, 7.5) with 0.15% DTT.
The preparation of standard solution and the derivatization of the −NH2 and −SH groups of cysteine to obtain their respective N,S-ethoxycarbonyl methyl esters was as previously described.28 Briefly, derivatization was performed using ethylchloroformate. After ethylacetate phase extraction, derivatization of the −COOH groups was performed with 1mM HCl in methanol. Dried samples and standards were dissolved in methanol for GC-MS analysis. For the preparation of standards, graded amounts of L-cysteine were added in phosphate buffer containing 1 nmol of L-[3,3-2H2]cysteine as an internal standard. Calibration curves were obtained by least-squares linear regression analysis of the L-cysteine to L-[3,3-2H2]cysteine peak area ratio versus the L-[3,3-2H2]cysteine molar quantity added to the sample. The intracellular cysteine concentration (expressed as nanomoles cysteine per million cells) was calculated using the slope (a), the intercept (b) of the cysteine calibration curve, and the cell count in the sample (c), corrected for the peak area ratio of cysteine at mass-to-charge ratio (m/z) 220 to that of L-[3,3-2H2]cysteine (referred to as 2H-Cysteine) at m/z 222:
GC-MS procedure
The concentrations of L-cysteine and L-[3,3-2H2]cysteine were determined using a gas chromatograph (5890 series II, Agilent Technologies) coupled with a quadrupole mass spectrometer (5971A; Agilent Technologies). Amino-thiols were separated on a fused poly (5% diphenyl/95% dimethylsiloxane) capillary column (HP-5MS, 30-m × 0.25-mm inner diameter, 0.25-μm film thickness; Agilent Technologies). The injector and detector temperatures were 270°C and 290°C, respectively, and the electron ionization source was held at 280°C. The column head pressure was set at 8 psi with a helium constant flow rate of 1.2 mL/min. Splitless injection was used. Injected volumes were 1 μL. The GC oven was programmed as follows: initial temperature 120°C hold for 0.5 minutes, ramp to 260°C at 10°C min−1, then to 290°C at 50°C min−1, hold for one minute. Selected ion monitoring mode with ions at m/z 220 and 222 for the N,S-ethoxycarbonyl methyl ester derivative of natural L-cysteine and L-[3,3-2H2]cysteine, respectively, was used to measure cysteine concentration. The ion dwell time was set at 100 ms.
DTH
LEW.1W rats were immunized subcutaneously using 200 μL of an emulsion of 50 μg of KLH mixed in PBS with 50% Freund complete adjuvant. DTH was induced by injecting intradermally 20 μL of a KLH solution (1 mg/mL) on a shaved area on the back, 7 days after immunization. Erythema swelling and induration were measured using a caliper within 24 to 72 hours. Rats were treated with PPG 50 mg/kg per day and NAL 500 mg/kg per day throughout the experiment.
Dinitrophenol-ovalbumin immunizations
Dinitrophenol-conjugated ovalbumin (DNP-Ova) was a gift from F. Nisol (University of Louvain, Louvain, Belgium). LEW.1W rats were immunized intraperitoneally with 10 μg of DNP-Ova. Nineteen days later, sera were collected by blood sinus puncture. Serum levels of anti-DNP IgG1 and IgG2a subclasses were determined by ELISA, as described previously.26 Briefly, 96-well microtiter plates (Immulon) were coated with bovine γ-globulin-DNP at 5 μg/mL and diluted sera added for 2 hours at 37°C. Plates were developed using horseradish peroxidase-conjugated mouse monoclonal anti–rat isotypes (IgG1, MARG1-2; IgG2a, MARG2a-1; University of Louvain). The concentration of anti-DNP antibody was estimated using standard curves generated by incubating the DNP-coated plates with anti-DNP rat monoclonal antibody (IgG1, LODNP-1; IgG2a, LODNP-16; University of Louvain).
Cytokine measurements
At day 5 or 100 after transplantation, transplants were harvested and homogenized with an Ultra-Turrax (Staufen) in RIPA buffer containing a protease inhibitor cocktail. The supernatant was stored at −20°C until cytokine content assessment. Intratransplant cytokine content and cytokine secretion from supernatant of MLR were assessed by ELISA according to the manufacturer's instructions: IFN-γ using OptEIA kit (BD Biosciences), IL-12 + p40 using ELISA kits (Invitrogen), and IL-23 and IL-27 using ELISA kits (Antibodies-online). The MoDC and THP-1 secretion of IL-12p40, IL-12p70, IL-10, and IL-6 in the supernatants was assessed by ELISA using OptEIA kits (BD Biosciences) according to the manufacturer's instructions.
Statistical analyses
Statistical significance was evaluated using Mann-Whitney tests for the comparison of 2 groups. Graft survival was evaluated by Kaplan-Meier analysis using the log-rank test.
Results
CSE is underexpressed in tolerated allografts
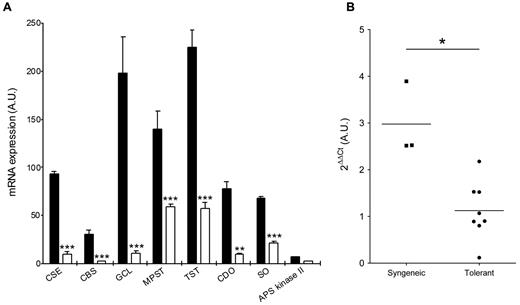
Microarray analysis of mRNA from transplanted rat kidney allografts revealed a consistent down-regulation of CSE and of several members of the trans-sulfuration pathway 4 months after transplantation in recipients that received a tolerizing regimen based on the administration of antidonor class II immune serum21 compared with syngeneic grafts (Figure 2A). In this kidney graft model, administration of nonimmune serum or of an irrelevant immune serum resulted in rejection within 2 weeks, instead of tolerance21 (and data not shown). Quantification by quantitative RT-PCR on independent samples showed that tolerated kidney allografts contained on average 3-fold less mRNA for CSE than syngeneic grafts (Figure 2B). A time-course experiment revealed that CSE down-regulation was visible already 4 days after transplantation and stayed low for at least 3 months compared with syngeneic grafted organs. However, an early drop in CSE mRNA was also observed in rejected grafts (supplemental Figure 1E-F). In the same kidney transplant model, a similar down-regulation of CSE was also observed if tolerance was induced by infusion with antagonist anti-CD28 antibodies, according to a previously described protocol22 (supplemental Figure 1B). After cardiac transplantation in the rat, tolerance can also be induced by donor-specific blood transfusions,23 administration of CD40Ig,24 or of the immunosuppressive molecule LF15-0195.29 In these cases, the down-modulation of CSE mRNA could also be observed in tolerant recipients (supplemental Figure 1C). In the donor-specific blood transfusion model, we could also measure a decrease of CSE enzyme activity in cardiac allografts (supplemental Figure 1D).
Reduced expression of CSE in transplant tolerance. (A) Tolerance is associated with a down-regulation of trans-sulfuration network genes. Gene regulation was assayed by microarray analysis at day 150 after transplantation in rats receiving an allogeneic kidney transplant and antidonor class II antibodies (empty bars) as the tolerizing regimen.21 Data are available on the Gene Expression Omnibus web-based data repository, under the GEO accession number GSE28474. APS Kinase indicates adenosine 5′-phosphosulfate kinase; CBS, cystathionine-β-synthase; CDO, cysteine dioxygenase; GCL, glutamate cysteine ligase; MPST, mercaptopyruvate sulfurtransferase; SO, sulfite oxidase; and TST, thiosulfate sulfurtransferase. Dara are mean ± SEM of 3 observations per group. **P < .01. ***P < .001. (B) Kidney transplants from rats receiving antidonor class II antibodies (Tolerant) and from syngeneic transplants (Syngeneic) were assayed for CSE expression by quantitative RT-PCR at day 100 after transplantation. Individual measurements are shown, and bars represent the mean value. *P < .05.
Reduced expression of CSE in transplant tolerance. (A) Tolerance is associated with a down-regulation of trans-sulfuration network genes. Gene regulation was assayed by microarray analysis at day 150 after transplantation in rats receiving an allogeneic kidney transplant and antidonor class II antibodies (empty bars) as the tolerizing regimen.21 Data are available on the Gene Expression Omnibus web-based data repository, under the GEO accession number GSE28474. APS Kinase indicates adenosine 5′-phosphosulfate kinase; CBS, cystathionine-β-synthase; CDO, cysteine dioxygenase; GCL, glutamate cysteine ligase; MPST, mercaptopyruvate sulfurtransferase; SO, sulfite oxidase; and TST, thiosulfate sulfurtransferase. Dara are mean ± SEM of 3 observations per group. **P < .01. ***P < .001. (B) Kidney transplants from rats receiving antidonor class II antibodies (Tolerant) and from syngeneic transplants (Syngeneic) were assayed for CSE expression by quantitative RT-PCR at day 100 after transplantation. Individual measurements are shown, and bars represent the mean value. *P < .05.
CSE inhibition prevented heart graft rejection
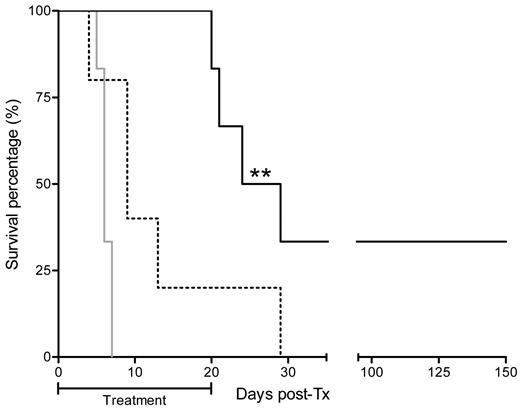
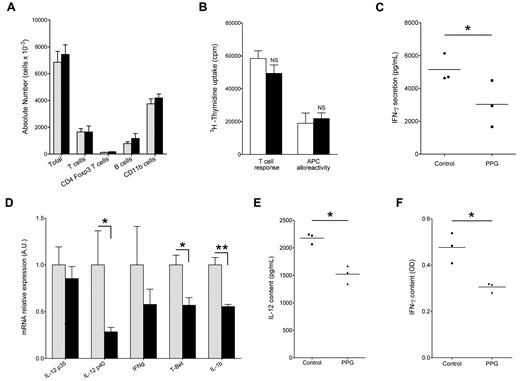
To test the effect of CSE inhibition on the heart graft rejection course, we injected the irreversible CSE inhibitor D,L-PPG on a daily basis over 20 days. PPG is known to inhibit the conversion of cysteine to H2S, as well as the conversion of homocysteine to cysteine, because CSE is implicated in these 2 conversion steps (Figure 1). Given that cysteine is the rate-limiting substrate for glutathione synthesis, a major ubiquitous antioxidant molecule, we supplemented PPG administration with a cysteine donor NAL, allowing us to block H2S release without blocking glutathione synthesis and therefore avoiding neuronal toxicity.30 Acute rejection of heart allograft in control animals consistently occurred on day 6 after transplantation, and the effect of administration of cysteine donor NAL alone was not significant (median survival, 9 days). By contrast, CSE inhibition by PPG supplemented with NAL induced a significant delay of rejection (median survival, 26.5 days). Furthermore, 2 of the 6 treated recipient animals tolerated their allograft indefinitely (Figure 3). Surprisingly, analyses performed on day 5 and 6 after transplantation by flow cytometry and immunofluorescence revealed that allografts were infiltrated with CD11b+ cells and T cells, to a similar extent in control or in PPG/NAL-treated recipients (Figure 4A and supplemental Figure 2A). On day 6 after transplantation, the ex vivo alloreactivity of recipient T cells against donor APCs in mixed lymphocyte reaction was normal, and not different between PPG/NAL treated animals and untreated control animals (Figure 4B). In unrelated mixed lymphocyte reactions, however, PPG showed some inhibitory capacity, of approximately 40%, on responding cells proliferation (data not shown). Recipient APCs also showed no defect in their capacity to elicit donor T-cell proliferation (Figure 4B), or third party T-cell proliferation (data not shown). However, donor T cells primed with APCs from treated rats produced less IFN-γ (Figure 4C). In addition, intragraft cytokine assessment on postoperative day 6 revealed a down-regulation of IL-12 and IFN-γ (Figure 4E-F). By RT-PCR, the lower expression of IL-12p40 was also visible (reduction of 71%) as well as IL-1β (45%) and the TH-1 transcription factor T-bet (43%), whereas IL-4, the TH-2, TH-17, and Treg transcription factors Gata-3, ROR-γT, and FoxP3, or other cytokines, including TGFβ and IL-12p35, were not modified (Figure 4D; supplemental Figure 2B). Graft-infiltrating cells extracted from 2 heart allografts also presented reduced mRNA levels of IL-12p40 but not of IL-10, i-NOS, or HO-1 (supplemental Figure 2C).
Impact of CSE blockade with PPG on cardiac allograft survival. The gray line represents survival of the rejection group without treatment (n = 6); dotted line, rats treated with NAL at 500 mg/kg per day alone (n = 5); and black line, rats treated with PPG at 50 mg/kg per day supplemented with NAL at 500 mg/kg per day (n = 6). **P < .01.
Impact of CSE blockade with PPG on cardiac allograft survival. The gray line represents survival of the rejection group without treatment (n = 6); dotted line, rats treated with NAL at 500 mg/kg per day alone (n = 5); and black line, rats treated with PPG at 50 mg/kg per day supplemented with NAL at 500 mg/kg per day (n = 6). **P < .01.
Immunologic status of cardiac allografts in PPG-treated animals. (A) Characterization of graft-infiltrating cells from NAL-treated (gray bars) or PPG + NAL-treated (black bars) animals. Graft-infiltrating cells were extracted at day 5 after transplantation, counted, and labeled with anti-TCRα/β, anti-CD4, and anti-FoxP3–labeled antibodies, or were stained with anti-CD11b, anti–class II, and anti-CD103 antibodies as described in “Immunostaining and flow cytometry.” Each bar represents the mean ± SEM of 3 animals. (B) “T cell response” panel: graft recipient (LEW.1A) T cells purified from the spleen were stimulated at a 1:1 ratio with irradiated donor-type LEW.1W splenocytes used as APCs. “APC alloreactivity” panel: graft recipient (LEW.1A) DCs from the spleen were used as APCs to stimulate at a 1:10 ratio purified donor-type LEW.1W T cells. White bars represent untreated rejecting recipients; and black bars, recipients treated with PPG + NAL. Cells were harvested on day 6 after transplantation. Proliferation was measured after 5 days of culture. Data are mean ± SD of triplicates from one experiment representative of 3 independent experiments. (C) At day 5 after transplantation, splenocytes from NAL-treated (Control) and PPG + NAL-treated (PPG) were used as APCs to stimulate allogeneic T cells in MLRs. Forty-eight hours later, supernatants were harvested and assessed for IFN-γ secretion. Individual measurements are shown with bars representing the mean. *P < .05. (D) Recipient rats of allogeneic hearts were either treated with NAL alone (gray bars) or with PPG + NAL (black bars). Grafts were harvested on day 5 after transplantation and mRNA levels of indicated factors analyzed by quantitative RT-PCR. Values for NAL alone controls were normalized at an arbitrary value of 1.0. Each bar represents the mean ± SEM of 4 (IFN-γ, IL-12p35, IL-12p40, IL-1b) or 5 (T-Bet) animals. *P < .05. **P < .01. (E-F) At day 5 after transplantation, NAL-treated (Control) and PPG + NAL-treated (PPG) transplants were homogenized, and IL-12 (E) or IFN-γ (F) content was assayed by ELISA in the soluble fraction. Individual measurements are shown with bars representing the mean. *P < .05.
Immunologic status of cardiac allografts in PPG-treated animals. (A) Characterization of graft-infiltrating cells from NAL-treated (gray bars) or PPG + NAL-treated (black bars) animals. Graft-infiltrating cells were extracted at day 5 after transplantation, counted, and labeled with anti-TCRα/β, anti-CD4, and anti-FoxP3–labeled antibodies, or were stained with anti-CD11b, anti–class II, and anti-CD103 antibodies as described in “Immunostaining and flow cytometry.” Each bar represents the mean ± SEM of 3 animals. (B) “T cell response” panel: graft recipient (LEW.1A) T cells purified from the spleen were stimulated at a 1:1 ratio with irradiated donor-type LEW.1W splenocytes used as APCs. “APC alloreactivity” panel: graft recipient (LEW.1A) DCs from the spleen were used as APCs to stimulate at a 1:10 ratio purified donor-type LEW.1W T cells. White bars represent untreated rejecting recipients; and black bars, recipients treated with PPG + NAL. Cells were harvested on day 6 after transplantation. Proliferation was measured after 5 days of culture. Data are mean ± SD of triplicates from one experiment representative of 3 independent experiments. (C) At day 5 after transplantation, splenocytes from NAL-treated (Control) and PPG + NAL-treated (PPG) were used as APCs to stimulate allogeneic T cells in MLRs. Forty-eight hours later, supernatants were harvested and assessed for IFN-γ secretion. Individual measurements are shown with bars representing the mean. *P < .05. (D) Recipient rats of allogeneic hearts were either treated with NAL alone (gray bars) or with PPG + NAL (black bars). Grafts were harvested on day 5 after transplantation and mRNA levels of indicated factors analyzed by quantitative RT-PCR. Values for NAL alone controls were normalized at an arbitrary value of 1.0. Each bar represents the mean ± SEM of 4 (IFN-γ, IL-12p35, IL-12p40, IL-1b) or 5 (T-Bet) animals. *P < .05. **P < .01. (E-F) At day 5 after transplantation, NAL-treated (Control) and PPG + NAL-treated (PPG) transplants were homogenized, and IL-12 (E) or IFN-γ (F) content was assayed by ELISA in the soluble fraction. Individual measurements are shown with bars representing the mean. *P < .05.
CSE modulates IL-12 biosynthesis and intracellular cysteine in monocytes and MoDCs
We tested the in vitro action of PPG on IL-12 synthesis by human MoDCs and monocytes after activation by LPS. In MoDCs, LPS stimulation resulted in the up-regulation of CD80, CD83, CD86, and HLA-DR and in the induction of IL-12 synthesis (Figure 5A). Using PPG during the maturation process, we found that IL-12 protein secretion was dose-dependently repressed (both IL-12 p40 subunit and IL-12 p70 complex were tested). In contrast, secretion of IL-6 or IL-10 was not affected by PPG (Figure 5B). The PPG dose range used here had no effect on cell viability (data not shown). The reduction of IL-12 synthesis was specifically the result of the inhibition of the CSE enzyme because repression could be reproduced by transfecting MoDCs with CSE siRNA that reduced CSE mRNA expression by 61% (data not shown) and IL-12 p40 secretion by 50% (Figure 5C). In addition, BCA, another CSE inhibitor, gave similar results (supplemental Figure 3). Other phenotypic characteristics of mature DCs, such as expression of CD80, CD86, CD83, and class II molecules were not modified by CSE inhibition (Figure 5A). The effect of CSE blockade on IL-12 production could not be attributed to the lack of H2S synthesis because simultaneous addition of NaHS (data not shown) or of GYY4137 (Figure 5D), 2 donor molecules that, respectively, release H2S rapidly or slowly, failed to restore IL-12 production. The NF-κB pathway, which is a known regulator of IL-12-p40 transcription,31 was obviously not involved either in the mechanism of action of PPG because no modification in the nuclear NF-κB binding activity could be observed in LPS-treated MoDCs after treatment with PPG (data not shown).
CSE inhibition prevents LPS-induced IL-12 synthesis by MoDCs and monocytes. (A) CSE inhibition does not prevent LPS-induced up-regulation of maturation markers. Human monocytes were differentiated for 5 days into MoDCs with IL-4 and GM-CSF, and maturation was induced by LPS for 24 hours with or without PPG (32mM) and analyzed by flow cytometry after gating on viable cells. Black bars represent LPS; and white bars, LPS + PPG. Data are from 3 independent experiments and are the mean ± SEM relative expression of indicated markers compared with immature MoDCs (gray bars). (B) Immature MoDCs were generated as in panel A, and maturation was induced by LPS for 24 hours, in the presence of increasing doses of PPG, after which time supernatants were analyzed by ELISA. Results represent the mean ± SEM relative cytokine secretion compared with secretion without PPG. ● represents IL-12p70 (n = 5); ■, IL-12-p40 (n = 10); ▴, IL-10 (n = 4); and itrif], IL-6 (n = 2) secretion. In these experiments, according to individual donors, IL-12p40 synthesis by LPS-treated MoDCs ranged from 7735 to 44 800 pg/mL, IL-12p70 from 481 to 31 300 pg/mL, IL-6 from 5185 to 14 600 pg/mL, and IL-10 from 5238 to 21 300 pg/mL. **P < .01. (C) Monocytes were differentiated during 4 days with IL-4 and GM-CSF, and transfected with an siRNA specific for CSE (siCSE) or a scrambled negative control siRNA (siC) for 24 hours Then, transfected cells were matured by LPS challenge for 24 hours, and supernatants were analyzed. Results represent the relative secretion of IL-12p40 compared with siC− and are the mean ± SEM of 4 independent experiments. In these experiments, LPS-treated MoDCs from the SiC- group secreted IL-12p40 from 270 pg/mL to 17 300 pg/mL. **P < .01. (D) Maturation of MoDCs was induced by LPS challenge for 24 hours in the presence of buffer (LPS), GYY4137 (GYY; H2S donor; 47μM), PPG, or combination of PPG (32mM) and GYY4137 (PPG + GYY), and supernatants were analyzed. Results represent the secretion mean ± SD of IL-12p40 (pg/mL) of duplicates from 1 experiment representative of 3 independent experiments. (E) Same experiment as in panel D, but SAS, a Xc− cystine transporter inhibitor, was added (500μM). Black bars represent IL-12p40; and white bars, IL-12p70. Results represent the mean ± SEM relative secretion of 4 independent experiments. In these experiments, LPS-treated MoDCs secreted IL-12p40 from 14 800 to 43 700 pg/mL and IL-12p70 from 480 to 21 300 pg/mL. *P < .05. (F) Same experiment as in panels D and E, in THP-1 cells. Results represent the mean ± SEM of 2 independent experiments. In these experiments, LPS-treated THP-1 secreted IL-12p40 from 231 to 370 pg/mL
CSE inhibition prevents LPS-induced IL-12 synthesis by MoDCs and monocytes. (A) CSE inhibition does not prevent LPS-induced up-regulation of maturation markers. Human monocytes were differentiated for 5 days into MoDCs with IL-4 and GM-CSF, and maturation was induced by LPS for 24 hours with or without PPG (32mM) and analyzed by flow cytometry after gating on viable cells. Black bars represent LPS; and white bars, LPS + PPG. Data are from 3 independent experiments and are the mean ± SEM relative expression of indicated markers compared with immature MoDCs (gray bars). (B) Immature MoDCs were generated as in panel A, and maturation was induced by LPS for 24 hours, in the presence of increasing doses of PPG, after which time supernatants were analyzed by ELISA. Results represent the mean ± SEM relative cytokine secretion compared with secretion without PPG. ● represents IL-12p70 (n = 5); ■, IL-12-p40 (n = 10); ▴, IL-10 (n = 4); and itrif], IL-6 (n = 2) secretion. In these experiments, according to individual donors, IL-12p40 synthesis by LPS-treated MoDCs ranged from 7735 to 44 800 pg/mL, IL-12p70 from 481 to 31 300 pg/mL, IL-6 from 5185 to 14 600 pg/mL, and IL-10 from 5238 to 21 300 pg/mL. **P < .01. (C) Monocytes were differentiated during 4 days with IL-4 and GM-CSF, and transfected with an siRNA specific for CSE (siCSE) or a scrambled negative control siRNA (siC) for 24 hours Then, transfected cells were matured by LPS challenge for 24 hours, and supernatants were analyzed. Results represent the relative secretion of IL-12p40 compared with siC− and are the mean ± SEM of 4 independent experiments. In these experiments, LPS-treated MoDCs from the SiC- group secreted IL-12p40 from 270 pg/mL to 17 300 pg/mL. **P < .01. (D) Maturation of MoDCs was induced by LPS challenge for 24 hours in the presence of buffer (LPS), GYY4137 (GYY; H2S donor; 47μM), PPG, or combination of PPG (32mM) and GYY4137 (PPG + GYY), and supernatants were analyzed. Results represent the secretion mean ± SD of IL-12p40 (pg/mL) of duplicates from 1 experiment representative of 3 independent experiments. (E) Same experiment as in panel D, but SAS, a Xc− cystine transporter inhibitor, was added (500μM). Black bars represent IL-12p40; and white bars, IL-12p70. Results represent the mean ± SEM relative secretion of 4 independent experiments. In these experiments, LPS-treated MoDCs secreted IL-12p40 from 14 800 to 43 700 pg/mL and IL-12p70 from 480 to 21 300 pg/mL. *P < .05. (F) Same experiment as in panels D and E, in THP-1 cells. Results represent the mean ± SEM of 2 independent experiments. In these experiments, LPS-treated THP-1 secreted IL-12p40 from 231 to 370 pg/mL
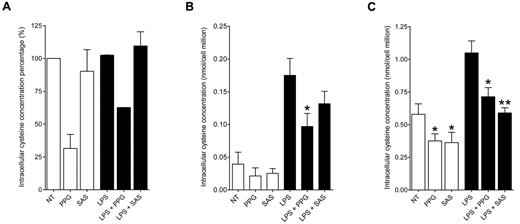
Because CSE is a rate-limiting enzyme in the synthesis of cysteine from methionine but also in the catabolism of cysteine into H2S, pyruvate, and NH3 (Figure 1), one major effect of CSE blockade might be an alteration of cysteine intracellular content. Because IL-12 synthesis is modulated by GSH,10 for which cysteine is limiting, we thought cysteine levels might also impact IL-12 synthesis. We therefore first tested whether SAS, an inhibitor of the cystine Xc− transporter, whose physiologic role is to import cystine, which then converts into cysteine, could mimic the effect of PPG on IL-12 synthesis in DCs. We observed that SAS prevented IL-12p40 subunit (as well as IL-12p70) production by LPS-stimulated MoDCs (Figure 5E). Next, we directly measured intracellular levels of cysteine by GC-MS in MoDCs, in freshly isolated monocytes, and in the THP-1 monocytic cell line, where CSE inhibition also prevents IL-12p40 secretion induced by LPS challenge (Figure 5F). In THP-1 cells, although there was no modification in cysteine content after LPS challenge compared with nontreated cells, CSE inhibition by PPG clearly decreased intracellular cysteine levels by 61% without LPS and by 27% with LPS (Figure 6A). Interestingly, in THP-1 cells, Xc− inhibition with SAS had no effect on cysteine levels (Figure 6A), in accordance with the observation that it could not prevent IL-12p40 secretion (Figure 5F), which highlights a crucial role for CSE activity in the replenishment of the cysteine content inside this cell line. In freshly isolated monocytes, low levels of cysteine were measured (0.04 nmol per cell million without LPS challenge) and LPS-induced maturation enhanced the intracellular cysteine content (0.18 nmol per cell million). Cysteine accumulation after LPS challenge was inhibited by 45% by PPG, whereas Xc− inhibition with SAS, even if it induced a moderate decrease, had no significant effect on cysteine levels (Figure 6B). Compared with monocytes, MoDCs presented higher intracellular cysteine levels (Figure 6C). In MoDCs, LPS challenge induced an up-regulation of intracellular cysteine content from 0.58 nmol to 1.05 nmol per million cells (Figure 6C), indicating that the immature state is associated with less cysteine content in MoDCs. CSE inhibition in MoDCs decreased intracellular cysteine content to 0.38 nmol without LPS and to 0.71 nmol per million cells with LPS. Unlike in THP-1 cells and fresh monocytes, Xc− inhibition with SAS clearly decreased the intracellular cysteine content of MoDCs, giving 0.36 nmol of cysteine without LPS and 0.59 nmol per cell million after LPS challenge (Figure 6C).
CSE inhibition prevents intracellular cysteine accumulation in monocytes and MoDCs. (A) THP-1 cells were nontreated (NT), treated with PPG (32mM), or treated with SAS (500μM) in the absence (white bars) or presence (black bars) of LPS for 24 hours. Cells were harvested and analyzed by mass spectrometry. Results represent the relative mean percentage of intracellular cysteine concentration compared with intracellular cysteine concentration when THP-1 cells were nontreated (NT; mean ± SEM of 2 independent experiments). In these experiments, NT cysteine levels ranged from 0.5 to 1.6 nmol of cysteine per million cells. (B) Same experiments as in panel A on freshly isolated monocytes. Results represent the mean of intracellular cysteine concentration, expressed as nanomoles of cysteine per million cells (mean ± SEM of 3 independent experiments). *P < .05. (C) Same experiments as in panel A on MoDCs. Results represent the mean of intracellular cysteine concentration, expressed as nanomoles of cysteine per million cells (mean ± SEM of 5 independent experiments, except SAS and LPS + SAS, 4 experiments). *P < .05. **P < .01.
CSE inhibition prevents intracellular cysteine accumulation in monocytes and MoDCs. (A) THP-1 cells were nontreated (NT), treated with PPG (32mM), or treated with SAS (500μM) in the absence (white bars) or presence (black bars) of LPS for 24 hours. Cells were harvested and analyzed by mass spectrometry. Results represent the relative mean percentage of intracellular cysteine concentration compared with intracellular cysteine concentration when THP-1 cells were nontreated (NT; mean ± SEM of 2 independent experiments). In these experiments, NT cysteine levels ranged from 0.5 to 1.6 nmol of cysteine per million cells. (B) Same experiments as in panel A on freshly isolated monocytes. Results represent the mean of intracellular cysteine concentration, expressed as nanomoles of cysteine per million cells (mean ± SEM of 3 independent experiments). *P < .05. (C) Same experiments as in panel A on MoDCs. Results represent the mean of intracellular cysteine concentration, expressed as nanomoles of cysteine per million cells (mean ± SEM of 5 independent experiments, except SAS and LPS + SAS, 4 experiments). *P < .05. **P < .01.
Because CSE is implicated in the cysteine synthesis as well as cysteine catabolism, these results indicate that the rate of CSE-induced cysteine synthesis in the trans-sulfuration pathway dominates the CSE-mediated cysteine catabolism in MoDCs and monocytes.
CSE inhibition prevents DTH but does not prevent antibody responses
Because we measured an effect of CSE on T-bet expression and IL-12 and IL-1β cytokines, which control TH-1 type immune responses,32 we investigated whether CSE blockade specifically blocked TH-1 type responses. DTH is a type IV hypersensitivity reaction mediated by TH-1 T lymphocytes.33 Sensitized animals challenged with an antigenic intradermoreaction against KLH mounted a robust skin DTH reaction. The reaction was not modified by administration of NAL. However, after administration of PPG + NAL, the reaction was mostly abolished (Figure 7). In contrast, animals immunized with ova-DNP mounted a specific antibody response (the prototypic TH-2 type of immune response), and this response could not be modified by administration of PPG + NAL (supplemental Figure 4).
CSE activity controls DTH. (A-B) LEW.1W rats were immunized with KLH and subsequently challenged with an intra-dermoreaction. Erythema (A) and induration thickness (B) were recorded at 24, 48, and 72 hours. ● represents untreated animals; ■, NAL-treated animals; and ▴, PPG/NAL-treated animals. Each point is the mean ± SEM of 4 animals, except for the PPG/NAL-treated group, which is the mean ± SEM of 7 animals. ***P < .001.
CSE activity controls DTH. (A-B) LEW.1W rats were immunized with KLH and subsequently challenged with an intra-dermoreaction. Erythema (A) and induration thickness (B) were recorded at 24, 48, and 72 hours. ● represents untreated animals; ■, NAL-treated animals; and ▴, PPG/NAL-treated animals. Each point is the mean ± SEM of 4 animals, except for the PPG/NAL-treated group, which is the mean ± SEM of 7 animals. ***P < .001.
Discussion
This study was initiated after we observed that CSE and several other genes of the trans-sulfuration pathway were repressed in different rat models of heart and kidney graft tolerance. Reciprocally, pharmacologic inhibition of CSE in vivo using PPG resulted in a significant prolongation of heart allogeneic graft survival. CSE blockade had some impact on T-cell responses in vitro in MLR, and spleen cells from PPG-treated animals elicited lower IFN-γ responses in contact with alloreactive T cells. In grafted animals, CSE blockade resulted in the repression of IL-12 synthesis, of T-bet, IFN-γ, and IL-1β within the graft, but factors such as GATA-3 and IL-4 were not modified. In vitro, PPG-treated DCs also presented a clear alteration of IL-12 production after activation. Other parameters, such as activation markers, immunogenicity, and secretion of IL-6 or IL-10, were not modified. Pertaining to this repression of IL-12, CSE inhibition prevented TH-1 type, but not TH-2 type, responses in vivo. In MoDCs, NF-κB activation or a direct action of the gasotransmitter H2S could not explain the effect of CSE inhibition on IL-12, which was associated with reduced intracellular levels of cysteine.
Transplant tolerance can be maintained by the action of regulatory lymphocytes and accessory cells that synergize to suppress allogeneic effector lymphocyte immunoreactivity.18 DCs are seen as dominant regulators of immune tolerance.34 However, although these cells have been evidenced as instrumental in some of these models,21 the underlying mechanism(s) are still unclear. An accepted common feature is the ability to present alloantigens in the presence of a suboptimal costimulation, resulting in alloreactive T-cell anergy, or differentiation into regulatory lymphocytes. Another feature is the alteration of cytokine synthesis that drives T-cell differentiation toward TH-1, TH-2, or Treg phenotypes. In 5 different models of organ transplant tolerance previously described in our laboratory,21-24,29 we found an underexpression of CSE. Therefore, CSE repression is probably a common mechanism independent of the tolerance induction protocol. However, a drop in CSE expression was also visible shortly after transplantation in rejected allografts from untreated recipients. That a factor associated with tolerance might also be present at rejection illustrates the coexistence of effector/regulatory mechanisms that determine immunologic fate of allografts.35,36 CSE is a key trans-sulfuration pathway enzyme that links methionine to cysteine and then controls its catabolism, resulting in the production of H2S. We initially thought that H2S might be responsible for the effect of CSE blockade on repression of IL-12 synthesis by DCs. Indeed H2S is now recognized as a biologically active gasotransmitter able to modulate production of cytokines and chemokines in leukocytes,37,38 in addition to the pleiotropic effects on the cardiovascular system.39 Moreover, the anti-inflammatory effect of CSE inhibition in vivo in the rat has been correlated to the induced reduction of H2S release,16 suggesting that H2S is the final mediator of the inflammatory effect. Expression of CSE has also been correlated with cell proliferation via a H2S-dependent mechanism.40 In DCs and THP-1 monocytes, however, IL-12 repression after inhibition of CSE could not be directly assigned to the effect of H2S deprivation because H2S supplementation failed to reverse the effect. Another argument against a direct role of H2S in the control of IL-12 transcription was that NF-κB binding activity in the nucleus was not modified by CSE blockade, whereas H2S has NF-κB inhibitory activity.14,38 Another possible mechanism by which CSE inhibition might repress IL-12 synthesis is via the alteration of GSH, which itself regulates IL-12 synthesis in macrophages by modulating the redox balance.8 However, GSH depletion inhibits NF-κB activation and prevents APCs from stimulating allogeneic T cells.41 Our observations are different: inhibition of CSE by PPG did not modify the expression of class II or costimulatory molecules on DCs and did not prevent their allogenicity. Therefore, it is probable that the effect of CSE blockade and cysteine reduction on IL-12 is independent of GSH. The intracellular cysteine level is controlled on one hand by the trans-sulfuration pathway and on the other hand by the import of cystine from the extracellular milieu. We observed that SAS, an Xc− inhibitor, also resulted in a repression of IL-12 synthesis in activated DCs. Therefore, these arguments, although not demonstrative, indicate that cysteine itself, presumably also by regulating the redox balance, could directly impact IL-12 transcription in DCs.
In vivo, the immunologic impact of CSE repression was mainly restricted to less IL-12, IFN-γ, IL-1β, and T-bet. T cell and APC alloreactivity ex vivo was normal, except that APC elicited T-cell responses producing less IFN-γ. Because MLR reactions could be to some extent modulated with PPG, we cannot exclude that the prevention of graft rejection might be the result of a direct effect of PPG on T-cell proliferation in vivo. However, because ex vivo alloreactivity of T cells was unaffected, the mechanism of action we favor to explain the delay in allograft rejection after PPG administration is the modification of cytokine synthesis of APC, resulting in the blockade of TH-1 type immune responses. Indeed, the key function of IL-12 is the induction and maintenance of TH-1 responses, which is essential in the antimicrobial responses to intracellular pathogens. DTH is a pure TH-1 type immune response that is dominantly controlled by IL-12. Antibody production is mediated by a TH-2 type T-cell help to B cells. Solid allograft rejection is mediated by both types of responses, but TH-2 alloresponses can also be protective.42 CSE blockade in vivo prevented DTH, did not affect antibody responses, and to some extent prolonged allografts with limited induction of transplant tolerance. These data are consistent with past reports suggesting that in some, but not all, models, reduced TH-1 responses induced by administration of neutralizing anti–IL-12 antibodies prolonged allograft survival.43-46 Interestingly, CSE mRNA levels were found reduced in tolerated allografts, suggesting a correlation between immune repression and CSE expression. The origin of the down-regulation in tolerated organs could be the result of the absence of graft-infiltrating cells expressing CSE. However, we observed that tolerated grafts were infiltrated by mononuclear cells to a similar extent as in rejected grafts and more infiltrated than syngeneic grafts. Another possible cause of CSE down-regulation in tolerated allografts might be a regulation of the transcription of the Cth gene (coding for CSE) by graft-infiltrating cells, which is transcriptionally controlled by the farnesoid X receptor, a bile acid-regulated nuclear receptor expressed in enterohepatic tissues and also in immune cells, such as macrophages.47 Previously, it was identified that reduced levels of CSE in human bone marrow may be a marker of cell immaturity.48 Because transplant tolerance is associated with intragraft infiltration by immature DCs,49 it is possible that the low CSE mRNA levels observed here simply reflect immaturity of graft-infiltrating cells expressing low CSE levels. The fact that we found that MoDCs in an immature state produce less intracellular cysteine than MoDCs matured with LPS, together with the role we uncovered for CSE in the replenishment of the cysteine pool in these cells, sustains this hypothesis. In addition, the previous finding that the presence of DC in spleen cells is required for adoptive transfer of tolerance from anti–class II antibody-treated rats21 stresses the central role of DCs in transplant tolerance. Whatever the mechanism responsible for low CSE expression in tolerated allografts, it is reminiscent of the TH-1 and IL-12 defects associated with HIV infection,50 characterized by sulfur loss and CSE underexpression,51 and for which administration of cysteine can increase anti-HIV responses.52,53
In conclusion, we found that CSE repression is associated with transplant tolerance. CSE specifically controls the intracellular cysteine level and IL-12 cytokine synthesis in DCs. In vivo, CSE controls TH-1 type immune responses and, as a coherent consequence, delays allograft rejection, pointing out the role of this enzyme in immune regulation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Francis Vanderbist from SMB-Galéphar (Brussels, Belgium) for providing Nacystelyn, Delphine Coulais from the Plateforme DTC (Centre Hospitalier Universitaire de Nantes, Nantes, France) for supplying monocytes, Charlotte Naël from the Plateforme de Spéctrométrie de masse for her advice regarding gas chromatography coupled with mass spectrometry, and Richard Danger for his DNA chip data management advice.
This work was supported by the Société Francophone de Transplantation, by the Vaincre La Mucoviscidose association, and by the Progreffe Fundation.
Authorship
Contribution: R.V.d.S. performed experiments and helped write the manuscript; F.C. and V.J. performed experiments; N.P., S.B., and G.B. discussed results; V.F.-R. designed GC-MS experiments; and B.V. founded research, designed experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernard Vanhove, Institut de Transplantation Urologie Nephrologie, Inserm Unité Mixte de Recherche-S 1064, Centre Hospitalier Universitaire Hôtel Dieu, 30 Bd Jean Monnet, 44093 Nantes, France; e-mail: bernard.vanhove@univ-nantes.fr.


![Figure 5. CSE inhibition prevents LPS-induced IL-12 synthesis by MoDCs and monocytes. (A) CSE inhibition does not prevent LPS-induced up-regulation of maturation markers. Human monocytes were differentiated for 5 days into MoDCs with IL-4 and GM-CSF, and maturation was induced by LPS for 24 hours with or without PPG (32mM) and analyzed by flow cytometry after gating on viable cells. Black bars represent LPS; and white bars, LPS + PPG. Data are from 3 independent experiments and are the mean ± SEM relative expression of indicated markers compared with immature MoDCs (gray bars). (B) Immature MoDCs were generated as in panel A, and maturation was induced by LPS for 24 hours, in the presence of increasing doses of PPG, after which time supernatants were analyzed by ELISA. Results represent the mean ± SEM relative cytokine secretion compared with secretion without PPG. ● represents IL-12p70 (n = 5); ■, IL-12-p40 (n = 10); ▴, IL-10 (n = 4); and itrif], IL-6 (n = 2) secretion. In these experiments, according to individual donors, IL-12p40 synthesis by LPS-treated MoDCs ranged from 7735 to 44 800 pg/mL, IL-12p70 from 481 to 31 300 pg/mL, IL-6 from 5185 to 14 600 pg/mL, and IL-10 from 5238 to 21 300 pg/mL. **P < .01. (C) Monocytes were differentiated during 4 days with IL-4 and GM-CSF, and transfected with an siRNA specific for CSE (siCSE) or a scrambled negative control siRNA (siC) for 24 hours Then, transfected cells were matured by LPS challenge for 24 hours, and supernatants were analyzed. Results represent the relative secretion of IL-12p40 compared with siC− and are the mean ± SEM of 4 independent experiments. In these experiments, LPS-treated MoDCs from the SiC- group secreted IL-12p40 from 270 pg/mL to 17 300 pg/mL. **P < .01. (D) Maturation of MoDCs was induced by LPS challenge for 24 hours in the presence of buffer (LPS), GYY4137 (GYY; H2S donor; 47μM), PPG, or combination of PPG (32mM) and GYY4137 (PPG + GYY), and supernatants were analyzed. Results represent the secretion mean ± SD of IL-12p40 (pg/mL) of duplicates from 1 experiment representative of 3 independent experiments. (E) Same experiment as in panel D, but SAS, a Xc− cystine transporter inhibitor, was added (500μM). Black bars represent IL-12p40; and white bars, IL-12p70. Results represent the mean ± SEM relative secretion of 4 independent experiments. In these experiments, LPS-treated MoDCs secreted IL-12p40 from 14 800 to 43 700 pg/mL and IL-12p70 from 480 to 21 300 pg/mL. *P < .05. (F) Same experiment as in panels D and E, in THP-1 cells. Results represent the mean ± SEM of 2 independent experiments. In these experiments, LPS-treated THP-1 secreted IL-12p40 from 231 to 370 pg/mL](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/11/10.1182_blood-2011-04-350546/4/m_zh89991287320005.jpeg?Expires=1767731932&Signature=WIJAaFsGESWi2lloIHpLWsg~LFAq9tCTvTGpX46iA1j7K5~oNzMDKzIMTg14jq9XZ0ykVcmluH0YHU2GbdrnrvNli185PdgOa1Kc8VuzoPSxZ-AIiHqzZUftdnMS7Jbic7Sl4k4TgnCX4Oai3m3FWE5yKFw3CFvnso2iB9kXWs9PKXo5Ug5ahIcCv6UwS6zOc4wow07kNHd3CFLkoWJVuhQvK-8W6OmTPl2YFULgjzhz5TZPH3CXOJHxuj~NlDCro6YyGJzPl4bC5THj3W501oyvL9SYTrJXmTdXzqJQ8MOmlQ1srpYI5cBt0e57fkBTDzuN-KjGbW9FbGYpT2NQ2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal