Abstract
Accumulating evidence indicates that infiltrating stromal cells contribute directly and indirectly to tumor growth in a wide range of cancers. In follicular lymphoma (FL), malignant B cells are found admixed with heterogeneous lymphoid-like stromal cells within invaded lymph nodes and BM. In addition, mesenchymal stromal cells (MSCs) support in vitro FL B-cell survival, in particular after their engagement toward lymphoid differentiation. We show here that BM-MSCs obtained from patients with FL (FL-MSCs) display a specific gene expression profile compared with MSCs obtained from healthy age-matched donors (HD-MSCs). This FL-MSC signature is significantly enriched for genes associated with a lymphoid-like commitment. Interestingly, CCL2 could be detected at a high level within the FL-cell niche, is up-regulated in HD-MSCs by coculture with malignant B cells, and is overexpressed by FL-MSCs, in agreement with their capacity to recruit monocytes more efficiently than HD-MSCs. Moreover, FL-MSCs and macrophages cooperate to sustain malignant B-cell growth, whereas FL-MSCs drive monocyte differentiation toward a proangiogenic and lipopolysaccharide-unresponsive phenotype close to that of tumor-associated macrophages. Altogether, these results highlight the complex role of FL stromal cells that promote direct tumor B-cell growth and orchestrate FL-cell niche, thus emerging as a potential therapeutic target in this disease.
Introduction
Tumorigenesis is widely recognized as a non–cell-autonomous process that depends on an intricate network of surrounding accessory supportive cells.1 Among this neoplastic microenvironment, cancer-associated fibroblasts (CAFs) are phenotypically and functionally distinct from their normal counterpart, thus providing a niche-based model of oncogenesis resulting from the dynamic coevolution of both cancer and stromal cells.2,3 CAF phenotype and molecular signatures are strongly heterogeneous but are generally maintained in culture, implying stable alterations. This heterogeneity could be associated in part to tumor type and localization but is also probably ascribed to the diverse origins of CAFs, which have been reported to derive from resident local fibroblasts, from BM-derived mesenchymal progenitors, or from passenger stromal cells brought with tumor cells. In particular, mesenchymal stromal cells (MSCs) could be recruited within tumors, where they incorporate into the stroma, become activated, and potentiate tumor growth in coinjection studies.4 MSCs have been shown to differentiate into CAF-like cells in vitro after exposure to cancer cell–conditioned medium5 and to stimulate tumor survival and proliferation, angiogenesis, and metastatic spread in experimental xenograft models.6,7 Even if few data are available on MSCs derived from patients with cancer, BM-MSCs from patients with multiple myeloma (MM) were shown to have a distinct molecular signature and to support more efficiently the growth of malignant plasma cells than BM-MSC obtained from healthy donors (HDs).8
Interestingly, it was recently demonstrated that solid tumors could direct the reorganization of surrounding stroma into lymphoid-like stromal structures involving fibroblastic reticular cell (FRC)–like cells.9,10 Within normal lymph node (LN), FRCs produce and ensheathe collagen bundles and other extracellular matrix components, building an enclosed conduit system involved in the delivery of small molecules, such as chemokines and antigens, to the T-cell zone. Moreover, FRCs provide a foothold for immune cell recruitment, motility, interaction, and homeostasis.11 We have previously demonstrated that LNs contained bona-fide MSCs and that human BM-MSCs as well as LN-MSCs could be committed to FRC differentiation in response to a combination of TNF-alpha and lymphotoxin alpha1beta2 (LT), the 2 main factors involved in the differentiation and maintenance of secondary lymphoid organs.12 Nevertheless, the precise relationships between MSCs, FRCs, and CAFs remain elusive.
Follicular lymphomas (FLs) are the most frequent indolent non-Hodgkin lymphomas and result from the transformation of germinal center (GC)–derived B cells retaining a strong dependence on their specific lymphoid microenvironment.13 In agreement, gene expression profiling (GEP) revealed that the outcome of patients with FL is primarily predicted by specific gene signatures of nonmalignant tumor-infiltrating cells.14 More recently, immunohistochemical studies have proposed a large panel of predictive markers reflecting the number, activation, and/or spatial distribution of infiltrating immune cells, in particular T cells and tumor-associated macrophages (TAMs).15
Concerning stromal cell niche in FLs, FRC meshwork is expanded and activated within invaded LNs,16 and in vitro functional studies have underlined that mesenchymal cells recruit malignant B cells and protect them from spontaneous and drug-induced cell death.12,17,18 In addition, stromal cells may also interact with FL-infiltrating immune cells to indirectly favor lymphoma progression. Interestingly, FL is generally a disseminated disease with initial involvement of both LN and BM.19 Moreover, BM infiltration is characterized by the ectopic development of lymphoid-like stromal cells of heterogeneous phenotype that are found admixed with malignant B cells.20 Whether these cells actually arise from in situ differentiation of resident MSCs is unknown. We previously showed that MSC-derived FRC-like cells display an increased capacity to sustain FL B-cell survival and that primary purified malignant B cells could promote FRC differentiation.12 These results suggest that malignant B cells can endow their stromal microenvironment with supportive properties. Within the BM, malignant B cells retained their main follicular features and underwent independent intraclonal evolution,21 suggesting that BM provides a preferred nonlymphoid tumor microenvironment that would be very useful in which to study stromal alterations.
To better understand characteristics and functions of stromal cells in FL, we investigated GEPs of BM-MSCs from patients with FL pertaining to lymphoid stroma signature and explored the functional implications of the specific FL-MSC signature. In particular, we highlighted the role of FL-associated stromal cells in the recruitment and polarization of monocytes into TAM-like cells. Combined with our previous studies, this work draw a more complete picture of how MSCs contribute to FL lymphomagenesis, through a dual role involving a direct B-cell supportive effect and an indirect activity on the orchestration of FL-cell niche.
Methods
Patients and cell samples
Subjects were recruited under Université de Rennes institutional review board approval, and informed consent process in accordance with the Declaration of Helsinki. BM aspirates were obtained from patients with FL at diagnosis and from HDs undergoing cardiac surgery. Clinical characteristics of the 10 patients with FL used to obtain FL-MSC GEP are listed in supplemental Table 1 (available at the Blood Web site; see the Supplemental Materials link at the top of the online article).
BM plasma were collected by centrifugation and frozen until use. BM mononuclear cells (BM-MNCs) were isolated by Ficoll density gradient and seeded at 104 cells/cm2 in α-MEM (Life Technologies) supplemented with 10% screened FCS (Hyclone), 100 IU/mL penicillin, 100 μg/mL streptomycin, and 1 ng/mL fibroblast growth factor 2 (FGF-2; R&D Systems).22 Nonadherent cells were discarded after 2 days of culture, and adherent cells were replenished twice a week with fresh culture medium until confluence (P0). BM-MSCs were then harvested with trypsin and seeded at 500 cells/cm2 until confluence (first passage, P1). The frequency of colony-forming unit-fibroblast (CFU-F) in BM-MNCs was evaluated in parallel for all MSC batches. At day 10, cells were stained with May-Grunwald-Giemsa, and colonies with > 50 cells were counted. The growth kinetic was assessed by the number of population doubling calculated as: Log (number of cells at the end of passage/number of seeded cells)/Log2, where the number of seeded cells for P0 was the number of CFU-F within BM-MNCs. The complete phenotype of all HD-MSC and FL-MSC batches was performed at the end of P1 before microarray experiments (see supplemental Figure 1). In addition, BM-MSCs were used at passages 2 to 3 for functional studies.
Primary malignant B cells from FL LNs were purified by negative selection as described.23 Peripheral blood monocytes were obtained by elutriation (J6 centrifuge; Beckman Coulter) followed by an additional depletion of residual T cells by the use of anti-CD3 microbeads (Miltenyi Biotec). Only cell fractions with at least 98% cell purity as evaluated by flow cytometry were used for further experiments. BL2, VAL, and RL GC-derived lymphoma B-cell lines were maintained in RPMI1640-10% FCS.
GEP study
GEP was performed on 10 BM-MSCs obtained from patients with FL (FL-MSCs) and from 6 age-matched HDs (HD-MSCs) treated or not for 3 days by TNF (10 ng/mL) and LT (100 ng/mL; R&D Systems) to induce their FRC-like differentiation.12 RNA was extracted with AllPrep ARN/ADN/protein kit (QIAGEN), and the purity and integrity of RNA were checked with the Bioanalyzer 2100 (Agilent). All samples used for microarray experiments displayed a RNA integrity number > 8.9. Biotinylated cRNA were amplified according to the small sample labeling protocol (TwoCycle amplification kit; Affymetrix) and hybridized on GeneChip HG-U133 Plus 2.0 oligonucleotide microarrays (Affymetrix). Data analysis was performed with Partek Genomics Suite (Partek) as described in supplemental Methods. The microarray data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE35331 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE35331).
Real-time quantitative PCR
cDNA synthesis was performed on 1 μg of total RNA with the Superscript II reverse transcriptase and random hexamers (Invitrogen). We used then specific Taqman Gene Expression Assays (supplemental Table 2) and the TaqMan Universal master mix (Applied Biosystems). GAPDH was determined as an appropriate internal standard gene. Gene expression was measured by use of the ABI Prism 7700 Sequence Detection System based on the ΔCT calculation method.
B-cell growth assays
After serum deprivation, BL2 (2.5 × 104 cells/mL), RL, or VAL (1.25 × 104 cells/mL) were seeded with low serum concentration in the presence or absence of a confluent MSC monolayer pretreated or not with TNF/LT for 3 days. When indicated, we used as feeder cells in vitro–differentiated macrophages, obtained by a 4-day culture of purified monocytes with 10 ng/mL M-CSF (R&D Systems) in the presence or absence of FL-MSCs (ratio 3/1). MSCs and macrophages were extensively washed before the 3-day coculture with B cells. Cells were pulsed with 1 μCi/well tritiated thymidine (3H-TdR; Perkin Elmer) for the last 16 hours of culture, harvested, and counted on a liquid scintillation analyzer. For primary FL samples, purified malignant B cells (7.5 × 105 cells/mL) were seeded in RPMI1640-10% FCS in the presence or absence of FL-MSCs and/or macrophages. After 7 days of culture, nonadherent and adherent cells were collected, pooled, and the absolute number of CD19/CD20posCD105negCD14neg TOPRO-3neg viable B cells was evaluated by the use of FlowCounts beads (Beckman Coulter).
B-cell proliferation and apoptosis
VAL and RL were cultured with low serum concentration alone or in the presence of confluent FL-MSCs, in vitro–differentiated macrophages, or both. After 24 hours, cell apoptosis was analyzed by the use of active caspase-3 staining gated on CD19/CD20pos B cells. In the same experiments, cell proliferation was analyzed after 3 days with BrdU staining. In brief, cells were pulsed with 10μM BrdU (BD Biosciences) for 30 minutes before cell harvesting and quantification of the percentage of BrdUpos cycling cells among CD19/CD20posactive caspase-3neg viable B cells.
CCL2 quantification
CCL2 was quantified by the use of the DuoSet ELISA kit (R&D Systems) in BM plasma, in the supernatants of HD-MSCs and FL-MSCs collected at the end of P1, and in the supernatant of HD-MSCs stimulated with TNF/LT or cocultured with RL, VAL, BL2, or purified primary B cells for 3 days.
To confirm the stromal origin of secreted CCL2 in B-cell/MSC coculture, both CD19/CD20posCD105negDAPIneg viable B cells and CD19/CD20negCD105posDAPIneg viable MSCs were sorted with a FACSAria cell sorter (Becton Dickinson) at the end of the coculture and used to assess CCL2 mRNA expression by real-time quantitative (RQ)–PCR.
In addition, HD-MSCs were cocultured with VAL and RL for 3 days before the collection of the supernatant and cell detachment by trypsin. After depletion of residual B cells with anti-CD45 Dynabeads (Invitrogen), HD-MSCs were seeded again in culture for 10 days at confluence. Supernatants were collected at days 3 and 10 for CCL2 quantification by ELISA.
Migration assay
Purified monocytes (105 cells/100μL) were added in RPMI1640-1% FCS (migration medium) to the upper compartment of Transwell chambers with 5-μm pore filters (Corning Costar). Lower chambers contained supernatants obtained after 4 days of culture in migration medium of FL-MSCs, HD-MSCs, or HD-MSCs prestimulated by RL or VAL B-cell lines for 3 days. When indicated, CCL2 was depleted from MSC supernatants. The absolute number of viable monocytes was quantified in the lower chamber after 1.5 hours with FlowCount beads.
Macrophage polarization
Purified monocytes (1.5 × 105 cells/cm2) were cultured alone or with MSCs (5 × 104 cells/cm2) in the presence of M-CSF. After 1 day of coculture, TNF and IL-10 were quantified in cell supernatants with the use of specific ELISA assays (R&D Systems and BD Biosciences, respectively). In parallel, cells were harvested, and the expression levels of HLA-DR, CD86, and CD14 were evaluated by flow cytometry on CD14posCD105negTOPRO-3neg cells as the ratio of the mean of fluorescence intensity compared with isotype-matched controls. In addition, after 7 days of coculture, cells were stimulated or not with 100 ng/mL lipopolysaccharide (LPS; InvivoGen) for 5 hours before cell-sorting of CD14posCD105negTOPRO-3neg–viable macrophages. RNA was then extracted for RQ-PCR experiments. When specified, the γ-secretase inhibitor DAPT (25μM; Calbiochem) was added to evaluate the role of the Notch pathway. In this case, cell supernatants were collected 18 hours after LPS stimulation for measurement of TNF by ELISA.
Statistical analyses
Statistical analyses were performed with Prism Version 5.0 software (GraphPad Software) using the Wilcoxon or Student t test for matched pairs or using the Mann-Whitney nonparametric U test as appropriate.
Results
FL-MSCs display a specific gene expression profile
BM-MSCs were successfully obtained from all 6 HD and 10 FL patients, and the 16 MSC batches displayed similar cell morphology and phenotype, including a lack of CD45, CD14, CD34, and CD31 together with a strong expression of CD73, CD90, and CD105 (supplemental Figure 1 and Table 1). Moreover, no contamination by CD19pos B cells could be detected at the end of P1. Because BM-MSC growth has been correlated with the age of donors,24 we selected age-matched HDs (Table 1). In these conditions, neither the CFU-F concentration in the BM nor the cumulative population doubling of BM-MSCs significantly differed between HDs and patients with FL.
MSC growth and phenotype
| . | Follicular lymphoma patients (n = 10) . | Healthy donors (n = 6) . | P . |
|---|---|---|---|
| Sex ratio, M/F | 8/2 | 4/2 | |
| Age (y)* | 58.5 (42-76) | 54.0 (30-78) | |
| BM CFU-F* | 52.5 (10.5-625) | 161.7 (23.5-506) | .3132 |
| PD* | |||
| PD at P0 | 13.3 (11-15.1) | 12.7 (10.2-14.6) | .8749 |
| PD at P1 | 5.1 (3-5.8) | 5.4 (4.3-6.1) | .3831 |
| Total PD | 18.1 (14.1-20.9) | 18 (16.3-19.5) | .8749 |
| Karyotype | 46, XX [30] (2) | 46, XX [30] (2) | |
| 46, XY [30] (8) | 46, XY [30] (4) | ||
| Phenotype at the end of P1† | |||
| CD45 | 0.2 (0-0.5) | 0.2 (0-0.2) | |
| CD19 | 0.1 (0-0.3) | 0 (0-0.2) | |
| CD105 | 99.9 (99.1-100) | 99.6 (99.3-100) | |
| CD90 | 99.8 (99.5-100) | 99.9 (99.8-100) | |
| CD73 | 99.8 (99.5-100) | 99.9 (99.5-100) |
| . | Follicular lymphoma patients (n = 10) . | Healthy donors (n = 6) . | P . |
|---|---|---|---|
| Sex ratio, M/F | 8/2 | 4/2 | |
| Age (y)* | 58.5 (42-76) | 54.0 (30-78) | |
| BM CFU-F* | 52.5 (10.5-625) | 161.7 (23.5-506) | .3132 |
| PD* | |||
| PD at P0 | 13.3 (11-15.1) | 12.7 (10.2-14.6) | .8749 |
| PD at P1 | 5.1 (3-5.8) | 5.4 (4.3-6.1) | .3831 |
| Total PD | 18.1 (14.1-20.9) | 18 (16.3-19.5) | .8749 |
| Karyotype | 46, XX [30] (2) | 46, XX [30] (2) | |
| 46, XY [30] (8) | 46, XY [30] (4) | ||
| Phenotype at the end of P1† | |||
| CD45 | 0.2 (0-0.5) | 0.2 (0-0.2) | |
| CD19 | 0.1 (0-0.3) | 0 (0-0.2) | |
| CD105 | 99.9 (99.1-100) | 99.6 (99.3-100) | |
| CD90 | 99.8 (99.5-100) | 99.9 (99.8-100) | |
| CD73 | 99.8 (99.5-100) | 99.9 (99.5-100) |
Standard karyotype was performed at the end of P1 by analyzing 30 RHG-banded metaphases. Total PD = cumulative number of PD (PD at P0 + PD at P1). BM CFU-F indicates number of CFU-F/106 BM mononuclear cells; MSC, mesenchymal stromal cells; and PD, population doubling.
Data are expressed as median (range).
Data are expressed as median percentage (range).
Challenging data suggested that CAFs undergo genetic alterations during tumor progression,3 and microvascular endothelial cells in B-cell lymphomas were reported to harbor lymphoma-specific chromosomal translocations.25 However, we observed no karyotypic abnormality in HD- or FL-MSCs, and FISH analysis for t(14;18) produced consistently negative results, including in FL-MSCs obtained from patients with t(14;18)pos malignant B-cell clones (supplemental Figure 2). Recent studies have demonstrated that senescent cells secrete a complex set of proinflammatory and tumor-promoting factors.26 However, HD-MSCs and FL-MSCs displayed no β-galactosidase staining at the end of P1 (data not shown) and expressed a very low level of CDKN2A, the gene coding for the MSC senescence marker p16INK4a (supplemental Figure 3), suggesting that they were not engaged into a senescence process.24
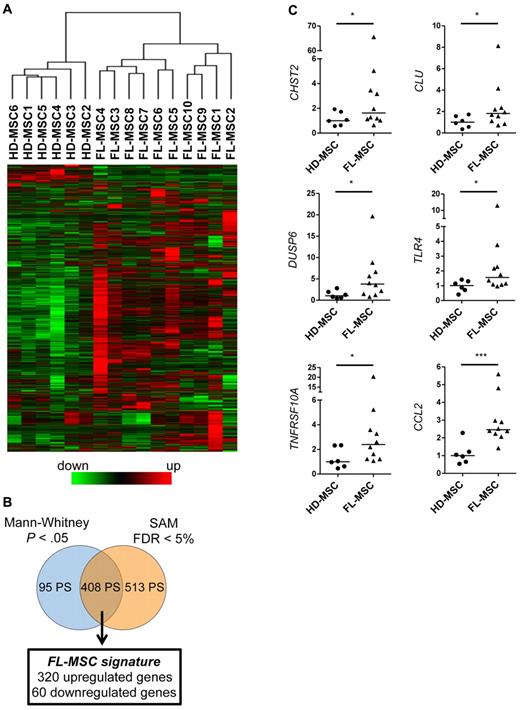
The GEP of resting HD-MSCs and FL-MSCs was determined at the end of P1 by the use of Affymetrix U133 Plus 2.0 microarrays. Raw data were normalized with the GC-RMA method and filtered to select the 9967 probesets (PS) showing the highest variation coefficient between samples. Strikingly, unsupervised hierarchical clustering analysis on this restricted dataset was able to perfectly segregate FL-MSCs from HD-MSCs (Figure 1A). To further delineate the specific GEP of FL-MSCs, we then performed a supervised analysis by combining an asymptotic unpaired Mann-Whitney U test and a SAM method. On the basis of this approach, we highlighted 408 PS representing the FL-MSC signature and corresponding to 320 up-regulated genes and 60 down-regulated genes (Figure 1B and supplemental Table 3). Among them, 16% were classified as coding for extracellular space or plasma membrane proteins (supplemental Figure 4). Interestingly, Ingenuity Pathway Analysis of this 408-PS list pointed out that the most significantly enriched biologic functions were linked to hematologic system development and function, hematopoiesis, and immune cell trafficking. The expression level of 6 genes was validated by RQ-PCR and yielded concordant results. For this specific analysis, we selected 2 genes coding for factors potentially implicated in B-cell adhesion and growth (CHST2 and CLU), 1 gene involved in cell proliferation and differentiation (DUSP6), and 3 genes regulating host/tumor interface (TNFRSF10A, TLR4, and CCL2). Among them, CCL2 was the most significantly differentially expressed genes between FL-MSCs and HD-MSCs (Figure 1C).
FL-MSCs display a specific gene expression profile. (A) Hierarchical clustering of resting untreated HD-MSCs (n = 6) and FL-MSCs (n = 10). Analysis was performed on the 9967 PS showing the greatest variation coefficient between samples. The relative level of gene expression is depicted according to the shown color scale. (B) Schematic representation of the statistical analysis used to highlight the FL-MSC signature defined as the intersection of the 2 genelists generated by SAM analysis (FC > 2 and false discovery rate < 5%) and Mann-Whitney rank test (FC > 2 or < 0.5 and P < .05). (C) Validation by RQ-PCR of the overexpression of CHST2, CLU, DUSP6, TLR4, TNFRSF10A, and CCL2 in FL-MSCs compared with HD-MSCs. Each sample was normalized to GAPDH, and the arbitrary value of 1 was assigned to the median expression of HD-MSCs. *P < .05; ***P < .001.
FL-MSCs display a specific gene expression profile. (A) Hierarchical clustering of resting untreated HD-MSCs (n = 6) and FL-MSCs (n = 10). Analysis was performed on the 9967 PS showing the greatest variation coefficient between samples. The relative level of gene expression is depicted according to the shown color scale. (B) Schematic representation of the statistical analysis used to highlight the FL-MSC signature defined as the intersection of the 2 genelists generated by SAM analysis (FC > 2 and false discovery rate < 5%) and Mann-Whitney rank test (FC > 2 or < 0.5 and P < .05). (C) Validation by RQ-PCR of the overexpression of CHST2, CLU, DUSP6, TLR4, TNFRSF10A, and CCL2 in FL-MSCs compared with HD-MSCs. Each sample was normalized to GAPDH, and the arbitrary value of 1 was assigned to the median expression of HD-MSCs. *P < .05; ***P < .001.
FL-MSCs are committed to a FRC-like phenotype
In FL, invaded BM is infiltrated by lymphoid-like stromal cells of heterogeneous phenotype and unknown origin and function. We thus decided to examine whether FL-MSCs actually displayed some common genetic features with FRCs. We first established the gene expression pattern of human FRC-like cells through the analysis of HD-MSCs treated by TNF/LT for 3 days, a stimulation process previously associated with the in vitro differentiation into functional FRC-like cells.12 After filtering the 8340 PS with the highest variation coefficient between samples, we underlined, by combining a paired t test and a SAM method, 1673 PS representing the TNF/LT signature and corresponding to 811 up-regulated genes and 431 down-regulated genes (Figure 2A and supplemental Table 4). The expression level of 7 genes identified by microarray strategy as highly induced by TNF/LT stimulation was validated by RQ-PCR (Figure 2B). Interestingly, TLR4 and CCL2 have been already pinpointed in the FL-MSC signature. Moreover, we also confirmed the up-regulation of IL6, HBEGF, IL15, IL8, and CCL5 that were all involved in the recruitment and/or activation of immune cells.
FL-MSCs are committed to a FRC-like phenotype. (A) Schematic representation of the statistical analysis used to highlight the TNF/LT signature defined as the intersection of the 2 genelists generated by SAM analysis (FC > 2 or < 0.5 and false discovery rate < 0.1%) and paired t test (FC > 2 or < 0.5 and P < .005). (B) Validation by RQ-PCR of the overexpression of TLR4, IL6, HBEGF, IL15, CCL2, IL8, and CCL5 in HD-MSCs stimulated by TNF/LT for 3 days compared with untreated HD-MSCs. Each sample was normalized to GAPDH and compared with expression in untreated HD-MSCs. The results are the mean ± SD obtained on 3 different HD-MSCs. (C) Gene set enrichment analysis enrichment score curves for the TNF/LT signature in MSC samples via use of the SNR statistic to rank the genes. Vertical black lines indicate the position of each of the 811 nonredundant up-regulated genes included in the TNF/LT signature, and the green curve represents the running sum of the weighted enrichment score. (D) Hierarchical clustering of HD-MSCs and FL-MSCs performed on the 1673 PS of the TNF/LT signature. The relative level of gene expression is depicted according to the shown color scale. (E) BL2 cell line was cultured in low serum concentration alone, or with confluent HD-MSCs (n = 9) or FL-MSCs (n = 8), pretreated or not with TNF/LT. Tritiated thymidine (3H-TdR) incorporation was evaluated at day 3. MSCs cultured alone always showed a 3H-TdR incorporation ≤ 500 cpm. **P < .01; ***P < .001
FL-MSCs are committed to a FRC-like phenotype. (A) Schematic representation of the statistical analysis used to highlight the TNF/LT signature defined as the intersection of the 2 genelists generated by SAM analysis (FC > 2 or < 0.5 and false discovery rate < 0.1%) and paired t test (FC > 2 or < 0.5 and P < .005). (B) Validation by RQ-PCR of the overexpression of TLR4, IL6, HBEGF, IL15, CCL2, IL8, and CCL5 in HD-MSCs stimulated by TNF/LT for 3 days compared with untreated HD-MSCs. Each sample was normalized to GAPDH and compared with expression in untreated HD-MSCs. The results are the mean ± SD obtained on 3 different HD-MSCs. (C) Gene set enrichment analysis enrichment score curves for the TNF/LT signature in MSC samples via use of the SNR statistic to rank the genes. Vertical black lines indicate the position of each of the 811 nonredundant up-regulated genes included in the TNF/LT signature, and the green curve represents the running sum of the weighted enrichment score. (D) Hierarchical clustering of HD-MSCs and FL-MSCs performed on the 1673 PS of the TNF/LT signature. The relative level of gene expression is depicted according to the shown color scale. (E) BL2 cell line was cultured in low serum concentration alone, or with confluent HD-MSCs (n = 9) or FL-MSCs (n = 8), pretreated or not with TNF/LT. Tritiated thymidine (3H-TdR) incorporation was evaluated at day 3. MSCs cultured alone always showed a 3H-TdR incorporation ≤ 500 cpm. **P < .01; ***P < .001
We next performed a gene set enrichment analysis approach to specifically assess the overrepresentation of the TNF/LT signature in FL-MSCs. Interestingly, we found that FL-MSC GEP was significantly enriched in genes overexpressed in HD-MSCs treated with TNF/LT (Figure 2C). In addition, among the 105 genes that were simultaneously found within the TNF/LT and the FL-MSC signatures, 101 were coordinately up-regulated or down-regulated in both HD-MSCs treated by TNF/LT and FL-MSCs (supplemental Figure 5 and supplemental Table 5). Finally, unsupervised hierarchical clustering revealed that the TNF/LT signature was sufficient per se to adequately segregate HD-MSCs from FL-MSCs (Figure 2D).
Because FL-MSCs and HD-MSCs have distinct molecular profiles, we performed functional coculture experiments to compare their respective capacity to reverse serum deprivation-induced growth arrest of GC-derived malignant B-cell lines. As previously reported,12 HD-MSCs efficiently sustained the growth of neoplastic B cells, and preliminary treatment by TNF/LT reinforced this feeder effect by 2-fold (P < .01; Figure 2E). Importantly, resting FL-MSCs exhibited a significantly better B-cell supportive activity than HD-MSC (13 310 cpm, range 7266-18 400 cpm vs 6663 cpm, range 5374-8264 cpm, P < .001), thus limiting the additional effect of TNF/LT prestimulation of FL-MSCs to a 1.26-fold increase. Altogether, these data argued for commitment of FL-MSCs toward a FRC-like phenotype consistent with their increased capacity to promote malignant B-cell growth.
Monocytes are specifically recruited by FL-MSCs and support FL cell growth
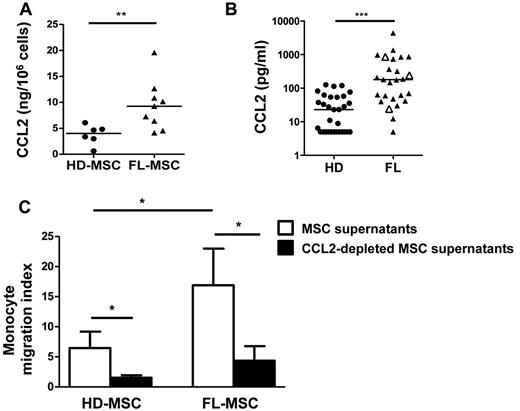
We decided to focus on CCL2 as the master up-regulated gene in FL-MSCs and a high-ranked member of the TNF/LT signature. We first demonstrated that CCL2 was produced at a higher level in the supernatant of FL-MSCs compared with HD-MSCs (Figure 3A). Moreover, we quantified CCL2 in the BM plasma from 26 FL patients and 31 HDs by ELISA and showed that CCL2 levels were variably but highly significantly increased in invaded BM compared with normal BM (474.4 pg/mL, range 5-4413 pg/mL vs 33.6 pg/mL, range 5-126.1 pg/mL; Figure 3B), indicating that CCL2 could play a role in vivo within malignant cell niche. Of note, the 3 patients with FL grade 3a did not display a different CCL2 level in BM plasma compared with the 23 patients with FL grade 1-2.
CCL2 is overexpressed by FL-MSCs and is involved in monocyte recruitment. (A) CCL2 production in culture supernatants from HD-MSCs (n = 6) and FL-MSCs (n = 9) was quantified by ELISA at the end of P1 and data are normalized by the number of cultured MSCs. **P < .01. (b) CCL2 was quantified in the BM plasma obtained from HDs (n = 31) and FL patients (n = 26, including 23 patients with FL grade 1-2 and 3 patients with FL grade 3a, identified as white triangles). ***P < .001. (C) Migration of purified peripheral blood monocytes in response to HD-MSC and FL-MSC supernatants collected at the end of P1 and specifically depleted or not from CCL2 with magnetic beads. Monocyte migration index is calculated as the number of TOPRO-3negCD14pos viable monocytes migrating in response to MSC supernatant divided by their number in response to migration medium. Results represent the mean ± SD from 3 (HD-MSCs) or 4 (FL-MSCs) independent experiments. *P < .05
CCL2 is overexpressed by FL-MSCs and is involved in monocyte recruitment. (A) CCL2 production in culture supernatants from HD-MSCs (n = 6) and FL-MSCs (n = 9) was quantified by ELISA at the end of P1 and data are normalized by the number of cultured MSCs. **P < .01. (b) CCL2 was quantified in the BM plasma obtained from HDs (n = 31) and FL patients (n = 26, including 23 patients with FL grade 1-2 and 3 patients with FL grade 3a, identified as white triangles). ***P < .001. (C) Migration of purified peripheral blood monocytes in response to HD-MSC and FL-MSC supernatants collected at the end of P1 and specifically depleted or not from CCL2 with magnetic beads. Monocyte migration index is calculated as the number of TOPRO-3negCD14pos viable monocytes migrating in response to MSC supernatant divided by their number in response to migration medium. Results represent the mean ± SD from 3 (HD-MSCs) or 4 (FL-MSCs) independent experiments. *P < .05
CCL2 had no direct impact on lymphoma B-cell chemotaxis or survival, as evaluated by the use of both GC-derived B-cell lines and primary purified FL B cells (data not shown). However, FL-MSCs recruited monocytes more efficiently than HD-MSCs (Figure 3C). To ascertain whether CCL2 contributed to FL-MSC–dependent monocyte migration, we specifically depleted CCL2 from MSC supernatants. Interestingly, CCL2 depletion abolished monocyte recruitment. Taken together, these results argued for a role of MSC-derived CCL2 on the cellular composition of FL microenvironment by attracting monocytes rather than for a direct activity on B-cell growth.
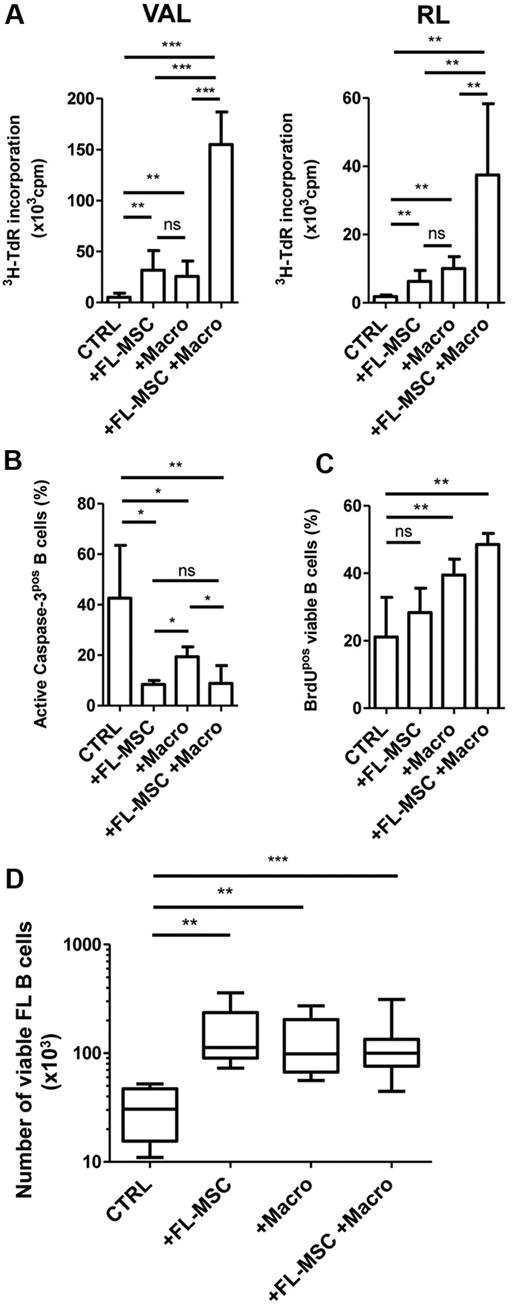
A high level of TAM has been correlated with a bad outcome in patients with FL who are treated with chemotherapy,27 but its involvement in FL B-cell growth has never been studied. We thus explored the growth-promoting activity of in vitro–differentiated macrophages on 2 GC-derived B-cell lines. Macrophages sustained the growth of VAL and RL cell lines in low serum concentration as efficiently as FL-MSCs. Moreover, FL-MSCs and macrophages, previously maintained in coculture for 4 days to allow their crosstalk in vitro, exhibited a synergistic activity on malignant B-cell growth (Figure 4A). To further unravel the mechanism of this synergy, we specifically investigated both the survival and the proliferative capacities of FL-MSCs, macrophages, and the combination of these 2 cell populations. Active caspase-3 staining revealed that FL-MSCs abrogated spontaneous VAL B-cell apoptosis, whereas the protective effect of macrophages was significantly lower (42.9% ± 18% for B cells alone vs 8.4% ± 1.5% with FL-MSCs vs 19.3% ± 3.9% with macrophages, P < .05, Figure 4B).
FL-MSCs and macrophages cooperate to sustain FL B-cell growth. (A-C) GC-derived B-cell lines were cultured in low serum concentration alone (CTRL), in the presence of FL-MSCs, in vitro–differentiated macrophages (Macro), or with a combination of Macro and FL-MSCs that have previously established a bidirectional crosstalk during a 4-day coculture. (A) Cell growth was evaluated at day 3 on VAL (left) and RL (right) by the incorporation of tritiated thymidine (3H-TdR). Results represent the mean ± SD from 6 experiments. MSCs and macrophages cultured without B cells always showed a 3H-TdR incorporation ≤ 500 cpm. (B) Apoptosis was evaluated at day 1 on VAL by the use of active caspase-3 staining gated on CD19/CD20pos B cells. Results represent the mean ± SD from 6 experiments. (C) Proliferation was evaluated at day 3 on VAL using BrdU staining gated on CD19/CD20pos B cells. Results represent the mean ± SD from 4 experiments. *P < .05; **P < .01; ***P < .001; ns: not significant. (D) Purified B cells obtained from 4 patients with FL were cultured alone (CTRL), in the presence of FL-MSCs, in vitro–differentiated macrophages (Macro), or with a combination of Macro and FL-MSCs that have previously established a bidirectional crosstalk during a 4-day coculture. The absolute number of B cells was assessed using TOPRO-3 staining and calibrated beads. **P < .01; ***P < .001.
FL-MSCs and macrophages cooperate to sustain FL B-cell growth. (A-C) GC-derived B-cell lines were cultured in low serum concentration alone (CTRL), in the presence of FL-MSCs, in vitro–differentiated macrophages (Macro), or with a combination of Macro and FL-MSCs that have previously established a bidirectional crosstalk during a 4-day coculture. (A) Cell growth was evaluated at day 3 on VAL (left) and RL (right) by the incorporation of tritiated thymidine (3H-TdR). Results represent the mean ± SD from 6 experiments. MSCs and macrophages cultured without B cells always showed a 3H-TdR incorporation ≤ 500 cpm. (B) Apoptosis was evaluated at day 1 on VAL by the use of active caspase-3 staining gated on CD19/CD20pos B cells. Results represent the mean ± SD from 6 experiments. (C) Proliferation was evaluated at day 3 on VAL using BrdU staining gated on CD19/CD20pos B cells. Results represent the mean ± SD from 4 experiments. *P < .05; **P < .01; ***P < .001; ns: not significant. (D) Purified B cells obtained from 4 patients with FL were cultured alone (CTRL), in the presence of FL-MSCs, in vitro–differentiated macrophages (Macro), or with a combination of Macro and FL-MSCs that have previously established a bidirectional crosstalk during a 4-day coculture. The absolute number of B cells was assessed using TOPRO-3 staining and calibrated beads. **P < .01; ***P < .001.
In contrast, when we considered the percentage of BrdUpos-viable B cells, we found that macrophages, unlike FL-MSCs, increased VAL B-cell line proliferation (Figure 4C). Similar results were obtained when RL cell line was used (data not shown). Primary FL B cells are poorly proliferating and highly prone to apoptosis in vitro. In accordance, we were able to confirm the survival potential of FL-MSCs and macrophages on malignant B cells purified from 4 unselected FL patients, but we could not detect any additional proliferative activity of the combination of both cell subsets (Figure 4D). Overall, these data supported the hypothesis that FL-MSCs and macrophages cooperated to sustain malignant B-cell growth through protection from apoptosis and enhancement of proliferation.
MSCs drive monocyte differentiation toward a TAM-like phenotype
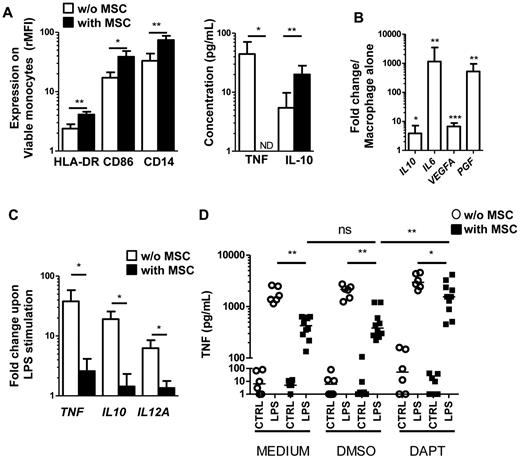
BM-MSCs affect the differentiation and function of dendritic cells and mature macrophages in vitro.28 In addition, umbilical cord blood–derived MSCs were recently reported to strongly reduce the T-cell stimulatory capacity of monocytes.29 We therefore studied the phenotype of monocytes cocultured for 24 hours and 7 days with FL-MSCs. After 24 hours, FL-MSCs increased the expression of HLA-DR, CD86, and CD14 on monocytes and modulated their secretory profile through a strong inhibition of TNF secretion together with an increased of IL-10 release (Figure 5A). In addition, IL-10, IL-6, and VEGFA, which have already been involved in the proangiogenic and protumoral activity of TAM in several tumor models, were up-regulated after 7 days of coculture in cell-sorted differentiated macrophages (< 0.5% MSC contamination, Figure 5B). Interestingly, we reported for the first time the strong induction (519-fold, range 23-1160, n = 9) of PGF, the gene coding for placental growth factor, a member of the VEGF family that binds VEGFR-1 and forms functionally active heterodimers with VEGF-A.30
FL-MSCs drive monocyte differentiation to a TAM-like phenotype. (A) Early modulation of monocyte phenotype and secretory profile was evaluated after 24 hours of culture with or without FL-MSCs. HLA-DR, CD86, and CD14 expression was evaluated by flow cytometry on CD14posCD105negTOPRO-3neg viable monocytes as the ratio of mean fluorescence intensity (rMFI) compared with isotype control (left). The production of TNF and IL-10 in the supernatant was simultaneously studied by ELISA (right). Results represent the mean ± SD from 5 experiments. *P < .05; ***P < .001; ND indicates not detectable. (B) Late modulation of macrophage gene expression profile was evaluated after 7 days of coculture of monocytes with or without FL-MSCs before cell sorting of CD14posCD105negTOPRO-3neg viable macrophages. Expression of IL10, IL6, VEGFA, and PGF was then evaluated by RQ-PCR. Each data were normalized to GAPDH and compared with expression in macrophages alone. The results are the mean ± SD from 9 experiments. *P < .05; **P < .01; ***P < .001. (C) Monocytes were cultured during 7 days with or without FL-MSCs before stimulation or not by LPS during 5 hours. CD14posCD105negTOPRO-3neg viable macrophages were then cell-sorted and expression of TNF, IL10, and IL12A was evaluated by RQ-PCR. Each sample was normalized to GAPDH and compared with expression in unstimulated macrophages. The results are the mean ± SD from 5 experiments. *P < .05. (D) Monocytes were preincubated or not (MEDIUM) with DAPT or its vehicle (DMSO) for 1 hour and cultured in the same conditions during 7 days with or without FL-MSCs. LPS was then added or not (CTRL) during 18 hours and TNF concentration was measured in cell supernatants by ELISA (n = 10). *P < .05; **P < .01; ns indicates not significant.
FL-MSCs drive monocyte differentiation to a TAM-like phenotype. (A) Early modulation of monocyte phenotype and secretory profile was evaluated after 24 hours of culture with or without FL-MSCs. HLA-DR, CD86, and CD14 expression was evaluated by flow cytometry on CD14posCD105negTOPRO-3neg viable monocytes as the ratio of mean fluorescence intensity (rMFI) compared with isotype control (left). The production of TNF and IL-10 in the supernatant was simultaneously studied by ELISA (right). Results represent the mean ± SD from 5 experiments. *P < .05; ***P < .001; ND indicates not detectable. (B) Late modulation of macrophage gene expression profile was evaluated after 7 days of coculture of monocytes with or without FL-MSCs before cell sorting of CD14posCD105negTOPRO-3neg viable macrophages. Expression of IL10, IL6, VEGFA, and PGF was then evaluated by RQ-PCR. Each data were normalized to GAPDH and compared with expression in macrophages alone. The results are the mean ± SD from 9 experiments. *P < .05; **P < .01; ***P < .001. (C) Monocytes were cultured during 7 days with or without FL-MSCs before stimulation or not by LPS during 5 hours. CD14posCD105negTOPRO-3neg viable macrophages were then cell-sorted and expression of TNF, IL10, and IL12A was evaluated by RQ-PCR. Each sample was normalized to GAPDH and compared with expression in unstimulated macrophages. The results are the mean ± SD from 5 experiments. *P < .05. (D) Monocytes were preincubated or not (MEDIUM) with DAPT or its vehicle (DMSO) for 1 hour and cultured in the same conditions during 7 days with or without FL-MSCs. LPS was then added or not (CTRL) during 18 hours and TNF concentration was measured in cell supernatants by ELISA (n = 10). *P < .05; **P < .01; ns indicates not significant.
We next decided to evaluate the responsiveness of macrophages maintained in culture with FL-MSCs to proinflammatory stimuli. Macrophages purified by cell-sorting after 7 days of coculture with FL-MSCs have a reduced capacity to express proinflammatory and anti-inflammatory cytokines in response to LPS (Figure 5C). Similar results were obtained with the use of HD-MSCs and FL-MSCs from patients with grade 1, grade 2, and grade 3a FL (supplemental Figure 6).
The Notch pathway regulates cell differentiation, proliferation, and survival in numerous contexts. Notch1-4 receptors interact with Notch ligands of the Jagged and Delta-like families, leading to the proteolytic cleavage of the Notch receptor by the γ-secretase, followed by the nuclear translocation of intracellular Notch domain. Recently, it has been reported that human BM-MSCs and adipose tissue-derived MSCs strongly express Jagged-1 and that Notch signaling is involved in their T-cell suppressive activity.31,32 To explore the involvement of the Jagged/Notch interaction in the unresponsiveness of MSC-treated macrophages to LPS stimulation, we added DAPT, a highly selective γ-secretase inhibitor, during macrophage/MSC coculture. As shown in Figure 5D, the addition of DAPT significantly enhanced the capacity of macrophages cultured with FL-MSCs to produce high amounts of TNF in response to LPS stimulation (1814 ± 1198 pg/mL with DAPT vs 530 ± 374 pg/mL with DMSO vs 442 ± 186 pg/mL with medium; n = 10). Interestingly, the production of IL-10 in response to LPS was also restored when the Notch pathway was inhibited during MSC/macrophage coculture (data not shown). HD-MSCs modulated similarly the response of macrophages to LPS stimulation (data not shown).
In summary, monocytes exhibited a CD14hiCD86hiHLA-DRhiTNFloIL-10hi phenotype after a short course coculture with FL-MSCs and HD-MSCs and differentiated in 7 days into macrophages expressing high levels of proangiogenic factors and unresponsive to LPS stimulation. These data were strongly in favor of a TAM-like polarization.
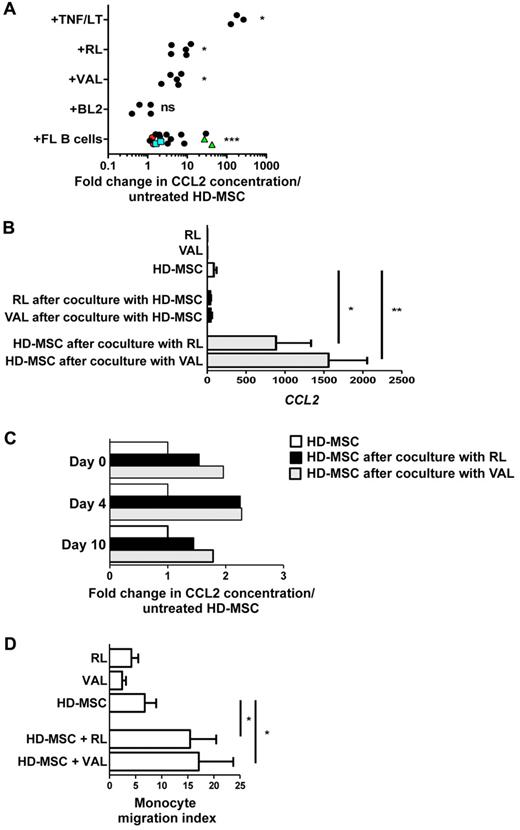
FL B cells promote CCL2 secretion by HD-MSCs
We next sought to assess whether malignant B cells could be involved in the overexpression of CCL2 by FL-MSCs. For that purpose, HD-MSCs were cocultured with TNF/LT as a positive control, as well as with various GC-derived B-cell lines and primary purified FL B cells. These experiments revealed an increased CCL2 secretion in the presence of malignant B cells, even if this induction was highly variable depending on the patient (from 1.1- to 42.3-fold, n = 16) and the cell line tested (Figure 6A). Interestingly, the coculture of the same primary FL B cells with 2 different HD-MSC batches revealed intrinsically different capacity of malignant B cells to induce CCL2 production in HD-MSCs with strong CCL2-inducers and poor CCL2-inducers B cells. To unequivocally identify the source of CCL2 within the coculture, HD-MSCs were cocultured with B-cell lines before cell-sorting of CD19/CD20posCD105neg B cells and CD19negCD105pos HD-MSCs.
FL B cells promote a prolonged CCL2 production by HD-MSCs. (A) HD-MSCs were stimulated for 3 days by TNF/LT (n = 3) or were cocultured with RL (n = 5), VAL (n = 5), or BL2 (n = 4) B-cell lines, or with purified primary FL B cells (n = 13). Three FL B cells were cocultured with 2 different HD-MSCs (green triangles, blue squares, and red circles, respectively). CCL2 concentration was then measured in cell supernatants by ELISA, and the results are expressed as the fold change compared with untreated HD-MSCs. *P < .05; ***P < .001; ns indicates not significant. (B) HD-MSCs were cultured for 3 days with RL or VAL before cell sorting of CD19/CD20posCD105negDAPIneg viable B cells and CD19/CD20neg CD105posDAPIneg viable MSCs and quantification of CCL2 by RQ-PCR in each cell fraction. Same experiments were conducted with HD-MSCs, RLs, and VALs cultured alone. Each CCL2 Ct value was normalized to matched GAPDH Ct value. The results are the mean ± SD from 3 experiments. *P < .05; **P < .01. (C) HD-MSCs were cultured for 3 days with RL or VAL before collection of cell supernatant (day 0). HD-MSCs were then detached, depleted from residual B cells, and seeded again in culture for 10 days. CCL2 concentration was measured in day 0, day 4, and day 10 cell supernatants by ELISA. Results are expressed as the fold change compared with untreated HD-MSC. Shown is 1 representative from 2 independent experiments. (D) Migration of purified peripheral blood monocytes in response to supernatants from RLs, VALs, HD-MSCs, and HD-MSCs maintained during 3 days in coculture with RL or VAL. Monocyte migration index is calculated as the number of TOPRO-3negCD14pos viable monocytes migrating in response to cell supernatant divided by their number in response to migration medium. Results represent the mean ± SD from 5 independent experiments. *P < .05
FL B cells promote a prolonged CCL2 production by HD-MSCs. (A) HD-MSCs were stimulated for 3 days by TNF/LT (n = 3) or were cocultured with RL (n = 5), VAL (n = 5), or BL2 (n = 4) B-cell lines, or with purified primary FL B cells (n = 13). Three FL B cells were cocultured with 2 different HD-MSCs (green triangles, blue squares, and red circles, respectively). CCL2 concentration was then measured in cell supernatants by ELISA, and the results are expressed as the fold change compared with untreated HD-MSCs. *P < .05; ***P < .001; ns indicates not significant. (B) HD-MSCs were cultured for 3 days with RL or VAL before cell sorting of CD19/CD20posCD105negDAPIneg viable B cells and CD19/CD20neg CD105posDAPIneg viable MSCs and quantification of CCL2 by RQ-PCR in each cell fraction. Same experiments were conducted with HD-MSCs, RLs, and VALs cultured alone. Each CCL2 Ct value was normalized to matched GAPDH Ct value. The results are the mean ± SD from 3 experiments. *P < .05; **P < .01. (C) HD-MSCs were cultured for 3 days with RL or VAL before collection of cell supernatant (day 0). HD-MSCs were then detached, depleted from residual B cells, and seeded again in culture for 10 days. CCL2 concentration was measured in day 0, day 4, and day 10 cell supernatants by ELISA. Results are expressed as the fold change compared with untreated HD-MSC. Shown is 1 representative from 2 independent experiments. (D) Migration of purified peripheral blood monocytes in response to supernatants from RLs, VALs, HD-MSCs, and HD-MSCs maintained during 3 days in coculture with RL or VAL. Monocyte migration index is calculated as the number of TOPRO-3negCD14pos viable monocytes migrating in response to cell supernatant divided by their number in response to migration medium. Results represent the mean ± SD from 5 independent experiments. *P < .05
RQ-PCR analysis confirmed the strong up-regulation of CCL2 in HD-MSCs, unlike in purified B-cell compartment (Figure 6B). We investigated whether induction of CCL2 production in HD-MSCs by malignant B cells was maintained in culture in the absence of continuous B cell–dependent stimulation. Interestingly, 10 days after B-cell removal, HD-MSCs retained an increased CCL2 production compared with unstimulated HD-MSCs, indicating that the effect of B cells was long-lasting (Figure 6C). Finally, we explored the functional relevance of this B cell–mediated CCL2 induction and demonstrated that conditioned media from HD-MSC/B-cell cocultures recruited more efficiently purified monocytes than unstimulated HD-MSC supernatant (Figure 6D).
Discussion
In this study, we identified the specific gene signature of BM-derived FL-associated stromal cells. We demonstrated that this signature is related to that of lymphoid-like stromal cells, suggesting an ectopic engagement to FRC differentiation in vivo. Interestingly the presence of lymphoid-like stromal networks has already been reported in murine models of solid cancers, where they contribute to the induction of an immunotolerant microenvironment.9,10 These cells are supposed to be locally induced during cancer-related inflammation, and an inflammatory gene signature has also been highlighted in fibroblasts obtained from mammary and pancreatic tumors in mice and humans, where they promote angiogenesis and inflammatory cell recruitment.33
Nevertheless, lymphoid-like differentiation is not a common feature of stromal cells in hematologic malignancies. In particular, the already-reported specific GEP of MM BM-MSC8 is not enriched for our FRC-like signature (data not shown), in agreement with the lack of morphologically detectable lymphoid-like structure in this disease. Importantly, to understand how malignant B cells could drive the emergence of an ectopic lymphoid-related stromal microenvironment, our study was performed on BM, unlike LN-MSCs. However, CCL2 is also overexpressed in stromal cells derived from FL LN compared with stromal cells derived from chronically inflamed tonsils (data not shown), suggesting a general role of CCL2 within stromal cell niche in FL that is not only related to cancer-related inflammatory process.
We have previously demonstrated that FRC-like cells generated in vitro by stimulation of BM-MSCs with TNF/LT are more powerful to drive malignant B-cell survival than BM-MSCs themselves.12 Our current results reinforce and extend these data because we could establish that FL-MSCs, already committed to a FRC-like differentiation, supported more efficiently the growth of malignant B cells. Similarly, MM-MSCs were shown to overstimulate the proliferation of a MM cell line compared with HD-MSCs.8 CCL2 had not direct impact on migration or survival of malignant FL B cells, in agreement with the repression of CCR2 expression by Pax5 B-cell transcription factor.34 The mechanisms of the direct tumor-promoting capacity of FL-MSCs remain thus to be identified. However, FL-MSCs also displayed indirect protumorigenic effects, including an increased capacity to recruit monocytes in a CCL2-dependent manner.
CCL2 is one of the most frequently observed chemokines in a wide range of solid cancers, where it harbors multifaceted activities by targeting both tumor cells and infiltrating myeloid cells. As an example, tumor-derived CCL2 recruits monocytes and promotes their survival and their polarization into CD14posCD206pos M2-type macrophages in prostate cancer.35 Moreover, CCL2 synthesized by both the tumor and the stroma in a mouse model of breast cancer metastasis was recently involved in the early recruitment of CCR2pos VEGFAhi inflammatory monocytes that contribute to the extravasation and seeding of tumor cells.36 In the same study, human inflammatory monocytes were shown to respond to the same CCL2-CCR2 axis for their recruitment into metastasis. Interestingly, it was recently suggested that perivascular BM stromal cells could sense circulating TLR ligands and, by producing CCL2, modulate the frequency of bloodstream inflammatory CCR2pos monocytes.37 Our work reveals that BM-MSCs could also induce CCL2-dependent monocyte migration in malignant context.
MSCs have been shown to produce a variety of anti-inflammatory mediators, including prostaglandin E2 and TNF-α–stimulated gene 6 (TSG-6/TNFAIP6), that promote macrophage reprogramming associated with the release of IL-10 and the decrease of inflammatory cytokines.29,38,39 In agreement, we demonstrate in our current study that HD-MSCs and FL-MSCs alter the phenotype and secretory profile of macrophages, leading to a polarization into TNFloIL-10hiVEGFAhiPGFhi LPS unresponsive TAM-like cells. Importantly, we highlight a key role for the Notch pathway in this process. In mouse macrophages, Notch activation promotes M1 polarization in response to LPS, a process supposed to be because of cooperation between Notch and NF-κB pathways.40 However, in human macrophages, the functional outcome of TLR and Notch interactions is more complex because Notch target genes feedback and attenuate TLR-induced cytokine production.41 In addition, human adipose tissue–derived MSCs mediate a Jagged-1 related inhibition of NF-κB signaling in T cells.32
Besides the induction of a proangiogenic and anti-inflammatory profile on macrophages, coculture of MSCs with macrophages give rise to a complex cell niche highly efficient in sustaining both survival and proliferation of malignant B cells. This synergy may be related to the crosstalk between the 2 cell partners, as recently suggested within hematopoietic stem cell niches where the interaction between macrophages and MSCs regulates hematopoietic stem cell retention.42 Multiple lines of evidence support the prominent role of TAM in the biology of FL, as highlighted by the poor predictive value of a high content of CD68pos or CD163pos cells in patients treated with chemotherapy.27,43 Moreover, the activation status of TAM is probably crucial in FL, as reported in several other cancers. In particular, the presence of STAT1pos TAM in FL was associated with an adverse outcome. Interestingly, the priming of macrophages with IFN-γ, which is overexpressed with FL microenvironment,44 was shown to mediate a STAT1-dependent induction of CCR2 associated with an increased migration in response to CCL2.45 Such activation loop should be important for the recruitment of macrophages in close vicinity to stromal cell/malignant B-cell aggregates. The precise mechanisms of the supportive activity of macrophages toward neoplastic B-cell remain unknown even if their production of B cell–activating factor of the TNF family (BAFF) and a proliferation inducing ligand (APRIL) have been recently involved in diffuse large B-cell lymphoma and gastric mucosa associated lymphoid tissue (MALT) lymphoma.46,47 Interestingly, we recently demonstrated that purified FL-TAM overexpress IL-15 that cooperates with T cell–derived CD40L signal to sustain FL cell growth.48 A detailed analysis of FL-TAM would be very interesting to further understand their role in lymphomagenesis.
A key unsolved issue is how tumor-infiltrating stromal cells acquire their protumoral phenotype and what could be the role of tumor cells in this process. In MM, adhesion of malignant plasma cells to BM stromal cells triggers the NF-κB–dependent release of various cytokines, including IL-6, which in turn favors tumor cell growth.49 Likewise, platelet-derived growth factor secreted by CLL B cells activates MSCs to proliferate and produce VEGF, thus indirectly regulating angiogenesis and CLL disease progression.50 Our results reveal that CCL2 is induced in HD-MSCs by coculture with malignant B cells, suggesting a bidirectional crosstalk between malignant B cells and stromal cells. We previously showed that FL B cells significantly overexpress TNF compared with normal tonsil B cells23 and the production of soluble TNF by normal B cells is essential for the proper organization of secondary lymphoid organ microarchitecture.51
Because TNF/LT also induced CCL2 expression in MSCs, it is tempting to speculate that TNF is, as least in part, involved in the up-regulation of CCL2 by FL B cells. In agreement, antagonist anti-TNF receptor I protein strongly reduces the malignant B cell–driven induction of CCL2 production by MSCs (supplemental Figure 7). The already-described wide disparity of TNF expression by malignant B cells23 could explain the strong variability of CCL2 induction in HD-MSCs by FL B cells and also could explain the heterogeneity of some functional data obtained in our study; however, we could not found any link, in our limited patient series, between FL grade 1 to 3a, which are now considered as equally indolent with indistinguishable clinical course,52 and either MSC properties or CCL2 concentration in FL BM. A detailed analysis of the genes induced in stromal cells by contact with malignant B cells in response to TNF-dependent versus TNF-independent pathways is in progress. Besides malignant B cells, several other cell subsets could contribute to the modulation of FL-TAM phenotype and function. In particular, we recently unraveled a strong infiltration of invaded FL LN by IL-4–producing follicular helper T cells (TFH).53 In addition to its potential role in B-cell survival and proliferation, IL-4 is also known as a key inducer of TAM phenotype.54 The presence and activation status of TFH or TFH-like cells within invaded BM are currently unknown.
Altogether, these data highlight the complexity of FL tumors where stromal cells directly promote tumor growth and act also as global organizers of FL cell niches through the recruitment and polarization of macrophages. Additional experiments that use purified FL-derived stromal cells obtained from invaded LN and BM would be helpful to address the specific role of MSCs and their highly heterogeneous lymphoid progeny in FL development and drug resistance. The identification and characterization of this intricate network of cell interactions may provide novel strategies to disarm the tumor-promoting functions of stromal cells and could have significant therapeutic potential as a complement to conventional antilymphoma drugs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christophe Ruaux for providing tonsil samples, Veronique Quillien for providing elutriated monocytes, the BREHAT network for FL bone marrow samples, Sophie Belal for her help in obtaining mesenchymal stem cells, the Institut Fédératif de Recherche (IFR)–140 of Rennes University for cell sorting core facility, and the Centre de Ressources Biologiques (CRB)–Santé of Rennes.
This work was supported by research grants from the Institut National du Cancer (INCa libre PL06-10 and PAIR Lymphome 2008-019), the Ligue Régionale Contre le Cancer, the Cancéropôle Grand Ouest, and the Association pour le Développement de l'Hémato-Oncologie (ADHO).
Authorship
Contribution: F.G. designed and performed research and analyzed data; G.C., C.M., P.A.-T., J.D., and C.H. performed research; C.P., J.D.V., and D.R. contributed to microarray experiments and statistical analysis; T.L. and O.F. organized sample collection and annotation; T.F. contributed to the study design; and K.T. designed and supervised research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karin Tarte, Inserm U917, Faculté de medicine, 2 Avenue du Pr Léon Bernard, 35043 Rennes, France; e-mail: karin.tarte@univ-rennes1.fr.