Abstract
HSCs undergo dramatic changes with aging. An increase in absolute numbers of HSCs along with a functional deficit in reconstitution potential and a shift toward production of myeloid cells are the hallmarks of murine hematopoietic aging. Here, we show that high levels of the inflammatory cytokine Rantes are found in the aging stem cell milieu. Forced overproduction of Rantes by retroviral expression in BM progenitors resulted in a deficit of T-cell output, and brief ex vivo exposure of HSCs to Rantes resulted in a decrease in T-cell progeny concomitant with an increase in myeloid progenitors. In contrast, Rantes knockout (KO) animals exhibit a decrease in myeloid-biased HSCs and myeloid progenitors and an increase in T cells and lymphoid-biased HSCs. KO HSCs retained their HSC subtype distribution and they produced more lymphoid-biased HSCs in transplantations. Rantes deficiency also resulted in a decreased mammalian target of rapamycin (mTOR) activity in KLS cells. In a heterochronic transplantation setting, we further show that aged HSCs placed in a young environment generate less myeloid cells. These data establish a critical role for environmental factors in the establishment of the aged-associated myeloid skewing phenotype, which may contribute to age-associated immune deficiency.
Introduction
HSCs are the source of the lifetime supply of all blood cells. In aged mice, the potency of HSCs diminishes, and animals experience a decline in immune function1 and increased incidence of myeloid malignancies.2 During normal aging, HSCs undergo dramatic changes both phenotypically and functionally. When quantified on the basis of phenotype, they have repeatedly been shown to expand with age,3-5 while their repopulating activity simultaneously decays,4,6,7 although there are some strain-specific behaviors.8,9 In addition, particular properties are altered; aged HSCs exhibit altered homing and mobilization properties10,11 and lymphoid cell production wanes, while myeloid cell production increases.4,6 The molecular mechanisms accounting for this constellation of change with age is not known, although environmental factors are thought to play a major role.12,13
Recently, several groups have demonstrated that the murine HSC compartment is heterogeneous, containing distinct HSC subtypes with different developmental preferences. The so-called myeloid-biased (My-bi) HSCs generate greater numbers of myeloid than lymphoid progeny, can contribute to blood production for exceptionally long periods of time, and have slower cycling kinetics. The lymphoid-biased (Ly-bi) HSCs more efficiently generate lymphoid cells, have shorter lifespans and have a faster turnover.9,14-17
Changes with age in the proportions of the HSC subtypes contributing to active blood production have recently been shown to at least partly underlie the predominance in myeloid cell production with age, with My-bi HSCs increasing dramatically with time.6,14,17 Again, the mechanism for the predominance of My-bi HSCs with age is not known. The My-bi HSCs may be better adapted to the aging niche or systemic environment. In the muscle, satellite cells, the stem cell of the muscle, have been shown to become biased in their differentiation toward fibrogenic lineages, mediated by increased levels of Wnt in the muscle of aged mice. This age-mediated bias can be reversed by exposing old cells to young milieu via parabiotic pairing between young and old mice,18,19 leading to great interest in defining the specific environmental factors that influence stem cells with age. A recent study identified the chemokine Ccl11 as increasing in serum with age, and as a contributor to the decline in neurogenesis in aged mice.20
For HSCs, investigation of the causes of lineage bias with age has primarily focused on cell-intrinsic changes.6,7 Studies on gene expression in aging purified HSCs showed significant dysregulation of many genes, particularly those genes associated with chromatin remodeling and inflammation.7 Up-regulation of inflammation-responsive genes may reflect the presence of an inflammatory environment in the aged BM where the HSCs reside. Such an environment may also impact stem cell survival and differentiation.
In this context, we analyzed the contribution of the environment to HSC differentiation and we set out to examine, in an unbiased fashion, changes in cytokines in the HSC niche that might account for some of the alterations associated with aging HSCs. We have identified the cytokine Rantes as a key player in murine aging HSC biology.
Methods
Mice
All mice were CD45.1 or CD45.2-C57Bl/6. Rantes KO (B6.129P2-Ccl5tm1Hso/J) were on a C57Bl/6 background backcrossed for 9 generations (The Jackson Laboratory). Mice were kept in a pathogen-free animal facility, and all procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Cytometric bead array
Cytokine levels in whole BM (WBM) were detected with a Cytokine Bead Array (BD Biosciences) and FacsArray plate reader (BD Biosciences).21 WBM supernatant was isolated from 2- and 24-month-old mice from tibias and femurs by centrifuging the bones in a 200-μL pipette tip at 500g for 8 minutes. The supernatant was pooled and protein quantified. After centrifuging the bones, the BM cells were flushed out and total cell numbers counted. Cells were maintained on ice during the whole procedure.
Retroviral transduction of progenitor cells, transplantation, and PB analysis
The mouse Rantes coding region was cloned into a murine stem cell virus (MSCV) vector and Sca-1–enriched 5FU-treated WBM was spin-infected.7 MSCV-Rantes-IRES-GFP– or MSCV-IRES-GFP–transduced cells were IV injected into irradiated (10.5 Gy) CD45.1 mice. Two to 3 mice were used as donors, and BM was transplanted into 10 recipients for each group. For peripheral blood (PB) analysis, mice were bled retro-orbitally, RBCs were lysed, and samples were stained on ice for 20 minutes with following Abs: CD45.2-allophycocyanin (APC), B220-PacBlue, CD4-PacBlue, CD8-PacBlue, B220-PE-Cy7, Mac-1-PE-Cy7, Gr-1-PE-Cy7. Cells were then analyzed with LSRII flow cytometer (BD Biosciences) in HBSS + 2% FBS (HBSS+) supplemented with propidium iodide (PI; 1 μg/mL).
Hematopoietic stem and progenitor analysis
WBM was isolated and stained with Hoechst and Abs as described.22-24 Briefly, BM cells stained at 1 × 106 cells/mL with 5 μg/mL Hoechst 33 342 for 90 minutes at 37°C in DMEM + 2% FBS. Cells were then washed and stained with Abs to identify donor-derived HSCs (SPKLS: SP, Lin−, Sca-1+, c-Kit+; SPKLS CD150+: SP, Lin−, Sca-1+, c-Kit+, CD150+), multipotent progenitor cells (Lin−, Sca-1+, c-Kit+, Flk2+) and KLS (Lin−, Sca-1+, c-Kit+). For lymphoid and myeloid progenitors, cells were stained with Ab schemes to identify donor-derived common myeloid progenitors (CMPs): Lin−, IL7rα−, Sca-1−, c-Kit+, FcγRlo; CD34+; granulocyte/monocyte progenitors (GMPs): Lin−, IL7rα−, Sca-1−, c-Kit+, FcγR+, CD34+; megakaryocyte/erythrocyte progenitors (MEPs): Lin−, IL7rα−, Sca-1−, c-Kit+, FcγR−, CD34−; and common lymphoid progenitors (CLPs): Lin−, IL7rα+, Sca-1lo, c-Kitlo, Flt3+. For Rantes knockout (KO) mice, same staining procedures were used.
Ex vivo treatment with Rantes
HSCs (SPKLS) were purified from 2-month-old mice (5 mice used as donors) then either treated or untreated with 30 ng/mL recombinant Rantes for 4 hours at 37°C. HSCs were transplanted into 2-month-old recipients (n = 7-8 mice/group) with 2 × 105 competitor WBM cells.
Heterochronic transplantation of WBM cells
WBM was isolated from either 2-month-old or 17- to 18-month-old (2 mice used as donors) and 2-2.5 × 106 WBM cells were transplanted into lethally irradiated 2-month-old and 17- to 18 month-old mice (n = 4-10 mice/group).
KO HSC transplantation
HSCs (SPKLS CD150+) were purified from 7- to 9-month-old WT or KO mice (1-2 mice) and transplanted into 2-month-old recipients (n = 5-10 mice/group) with 2 × 105 competitor WBM cells. For transplantations into the KO mice, HSCs (SPKLSCD150+) were purified from 17- to 18-month-old 45.1 mice (2 mice) and 250 HSCs were transplanted into 2- to 3-month-old WT or KO recipients with 2 × 105 competitor WBM cells (n = 6 WT, n = 7 KO).
Complete blood counts
Mice were bled using EDTA-coated tubes and analyzed on Hema-Vet 950 FS machine (Drew Scientific).
Quantitative real-time PCR
For bone-derived stromal cells: BM was removed by flushing, the bones were crushed and the slurry treated with collagenase/dispase for 30 minutes at 37°C; cells were washed and spun down at 500g. Media was removed, bone fragments vortexed, washed, and filtered (70μM). RNA was isolated from bone-derived stromal cells as well as from FACS-sorted KLS, SPKLS-CD150+ (HSCs) and lineage+ cells with RNAqueous. RNA was reverse-transcribed with random primers and with Super Script II (Invitrogen). TaqMan master mix (Applied Biosystems), 18S− rRNA probe (VIC-MGB; Applied Biosystems) and a gene-specific probe (FAM-MGB; Applied Biosystems) were used to perform RT-PCRs with an AbiPrism 7900HT (50 cycles). Normalization was made with 18S and fold change was calculated by DDCt (Delta delta Ct) method.
Intracellular staining
BM cells were stained for KLS markers then fixed with fix/lyse buffer (BD Biosciences), washed with PBS + 1% BSA, followed by incubation with Perm Buffer IV (BD Biosciences), then washed and stained with phospho–mammalian target of rapamycin (p-mTOR; Ser2448), p-S6 (Ser 235/236; Cell Signaling Technologies) Abs. After washing, Alexa Fluor 648–conjugated secondary Abs used. An LSRII (BD Biosciences) was used to analyze the samples.
Statistical analyses
Used Prism software, a 2-tailed t test was used where P values are indicated. A value of P < .05 was taken as significant.
Results
Transplantation of old BM cells into a young environment causes a decrease in myeloid cells
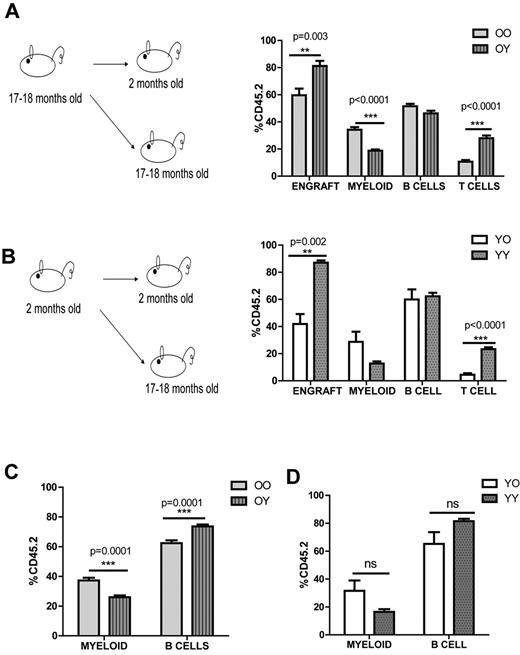
First, we asked how the environment affects donor cells from old mice and tested this by heterochronic transplantation. We isolated BM from 17- to 18-month-old mice and transplanted it into either 2-month-old or 17- to 18-month-old mice (old→young vs old→old; Figure 1A). Analysis of peripheral blood (PB) revealed a striking difference in engraftment and donor-derived myeloid and T lymphocytes. Old cells, when transplanted into young animals, generated fewer myeloid cells (18.8% ± 1% vs 34.2% ± 2%) and more T cells (26.1% ± 1.2% vs 10.6% ± 1%). The overall engraftment efficiency of old→old transplants was also significantly reduced (59.7% ± 4.9% vs 81.2% ± 3.8%; Figure 1A).
Young environment does not support myeloid development. (A) Graphical representation of old into young (O→Y) vs old into old (O→O) transplantation. WBM cells (2-2.5 × 106) from 17- to 18-month-old CD45.2 mice were transplanted into either 2-month-old (defined as O→Y) or 17- to 18-month-old (defined as O→O) CD45.1 mice. Lineage distribution of donor-derived cells of O→O vs O→Y transplants at 16 weeks (n = 10 O→Y, n = 8 O→O). (B) Graphical representation of young into young (Y→Y) vs young into old (Y→O) transplantation. WBM cells (2-2.5 × 106) from 2-month-old CD45.2 mice were transplanted into either 2-month-old (defined as Y→Y) or 17- to 18-month-old (defined as Y→O) CD45.1 mice. Lineage distribution of donor-derived cells of Y→O vs Y→Y at 16 weeks (n = 4 Y→Y, n = 7 Y→O). (C) Graph represents lineage distribution of donor-derived cells of O→O vs O→Y transplants at 16 weeks excluding T cells (n = 10 O→Y, n = 8 O→O). (D) Graph represents lineage distribution of donor-derived cells of Y→O vs Y→Y transplants at 16 weeks excluding T cells (n = 4 Y→Y, n = 7 Y→O). Error bars represent SEM. Representative of 2 experiments.
Young environment does not support myeloid development. (A) Graphical representation of old into young (O→Y) vs old into old (O→O) transplantation. WBM cells (2-2.5 × 106) from 17- to 18-month-old CD45.2 mice were transplanted into either 2-month-old (defined as O→Y) or 17- to 18-month-old (defined as O→O) CD45.1 mice. Lineage distribution of donor-derived cells of O→O vs O→Y transplants at 16 weeks (n = 10 O→Y, n = 8 O→O). (B) Graphical representation of young into young (Y→Y) vs young into old (Y→O) transplantation. WBM cells (2-2.5 × 106) from 2-month-old CD45.2 mice were transplanted into either 2-month-old (defined as Y→Y) or 17- to 18-month-old (defined as Y→O) CD45.1 mice. Lineage distribution of donor-derived cells of Y→O vs Y→Y at 16 weeks (n = 4 Y→Y, n = 7 Y→O). (C) Graph represents lineage distribution of donor-derived cells of O→O vs O→Y transplants at 16 weeks excluding T cells (n = 10 O→Y, n = 8 O→O). (D) Graph represents lineage distribution of donor-derived cells of Y→O vs Y→Y transplants at 16 weeks excluding T cells (n = 4 Y→Y, n = 7 Y→O). Error bars represent SEM. Representative of 2 experiments.
We then considered what impact the environment would have on the young donor cells and performed BM transplantation from 2-month-old mice into either 2-month-old or 17- to 18-month-old mice (young→young vs young→old; Figure 1B). Similarly, young cells when transplanted into old animals generated fewer T cells (4.4% ± 1.2% vs 20.8% ± 1.1%) and their engraftment (41.9% ± 7.4% vs 87% ± 1.6%) was reduced significantly. Although myeloid cells were increased in young→old transplants, it was not significant (28.7% ± 7.5% vs 12.7% ± 1.5%; Figure 1B). We also observed significantly fewer B cells when we compared old→young versus young→young or old→old versus young→old which was consistent with previous findings6 (Figure 1A-B). The defect in T cells when we transplanted either old or young cells into old environment was reported previously and could be because of the thymus involution in aged mice6 When we excluded T cells, we still observed a significant reduction in myeloid cells in the old→young compared with the old→old transplants (37.2% ± 1.9% vs 26% ± 1.3%; Figure 1C). Young cells generated similar numbers of myeloid cells in both old and young environments, even when we excluded the T cells (31.5% ± 7.4% vs 16.5% ± 1.9%), suggesting a role for intrinsic properties of the young cells (Figure 1B-D). The engraftment efficiency of both old→old and young→old transplants were decreased, further supporting the concept of a negative impact of the aged environment (Figure 1A-B). Thus, both environmental changes and intrinsic properties have a role in HSC differentiation potential during the aging process.
The intrinsic properties of old HSCs could be because of the compositional change of the HSC compartment, thus we decided to analyze the upper and lower SP (side population; lymphoid- and myeloid-biased populations, respectively) in 2- to 3-month-old vs 22- to 24-month-old mice. As we previously reported,17 cells with a higher Hoechst dye efflux ability were shown to be My-bi (lower-SPKLS) whereas Ly-bi stem cells (upper-SPKLS) were identified with a lower Hoechst efflux. In aged mice, My-bi lower-SPKLS cells accumulate in the BM. Although CD150 expression can be used to enhance this discrimination,17,25 here, we first selected KLS-CD150+ cells to identify the longest-term most conservatively defined HSCs, and then analyzed the upper and lower SP (lymphoid- and myeloid-biased populations, respectively). From this analysis, we observed that old mice had a significantly higher percentage of My-bi lower-SPKLS-CD150+ (63.5% ± 1.6% vs 83.3% ± 1%) and significantly lower percentages of Ly-bi upper-SPKLS-CD150+ (10.4% ± 1.2% vs 5.1% ± 0.6%) which was consistent with previous reports12,14-16 (supplemental Figure 1A-C, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The shift within KLS-CD150+ cells could be explained by a model in which a young environment enables balanced maintenance of both Ly-bi and My-bi HSCs, which in turn impacts development of myeloid progeny. In an old environment, this balanced state is not maintained because of a change in the composition of the stem cell milieu. Hence, Ly-bi HSCs are reduced in number whereas My-bi HSCs become more prevalent, which results in overproduction of myeloid cells.
Rantes protein and RNA levels increase with age in the BM
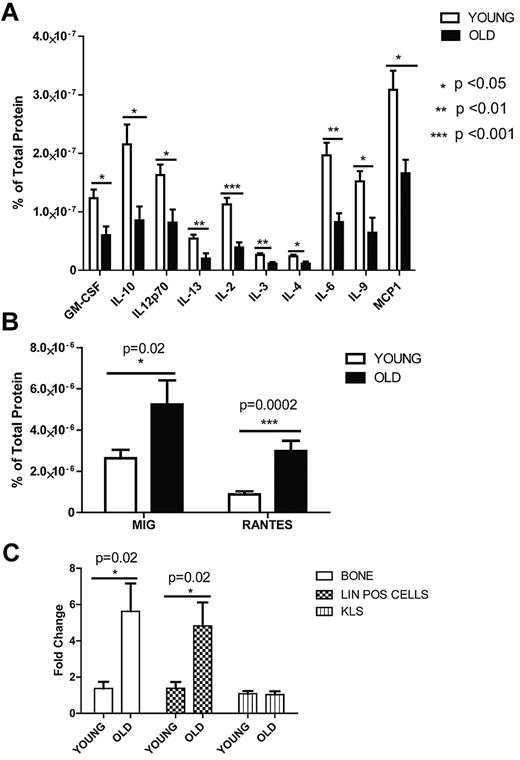
To begin to investigate the mechanism through which the old environment influences HSC composition and function, we first considered whether levels of particular cytokines, secreted proteins known to regulate the balance of many hematopoietic cell types, changed with age in the BM. We used a cytometric bead array to survey 17 different cytokine concentrations. BM supernatant was isolated from 2- and 24-month-old mice and a custom cytometric bead array was performed. Of 17 different cytokines, almost all declined in concentration with age (Figure 2A), except two that were higher in old mice; these were monokine induced by IFN-γ (Mig) and Rantes (Figure 2B). Both cytokines previously have been studied primarily in the context of T cells, where they are known to serve as chemoattractants and as promoters of inflammation.26
Cytometric bead array on aged and young BM andreal-time PCR analysis for Rantes expression in old and young BM populations. Cytokine levels in the BM were detected with a mouse cytokine bead array and FacsArray plate reader (both from BD Biosciences). We purchased each cytokine's cytometric bead array kit from BD Biosciences which can detect picogram levels of protein in serum or cell-culture supernatant (Flex set; BD Biosciences) and used in a multiplex manner. (A) Graph represents protein levels of cytokines either in the old or young BM supernatant normalized to total protein levels. (B) Graph represents Mig and Rantes protein levels either in the old or young BM supernatant (n = 10 young, n = 6 old). (C) Quantitave real-time PCR analysis of Rantes on mRNA purified from old or young bone cells, lineage-positive and hematopoietic stem-progenitor (KLS) cells (n = 3 young, n = 3 old). Error bars represent SEM.
Cytometric bead array on aged and young BM andreal-time PCR analysis for Rantes expression in old and young BM populations. Cytokine levels in the BM were detected with a mouse cytokine bead array and FacsArray plate reader (both from BD Biosciences). We purchased each cytokine's cytometric bead array kit from BD Biosciences which can detect picogram levels of protein in serum or cell-culture supernatant (Flex set; BD Biosciences) and used in a multiplex manner. (A) Graph represents protein levels of cytokines either in the old or young BM supernatant normalized to total protein levels. (B) Graph represents Mig and Rantes protein levels either in the old or young BM supernatant (n = 10 young, n = 6 old). (C) Quantitave real-time PCR analysis of Rantes on mRNA purified from old or young bone cells, lineage-positive and hematopoietic stem-progenitor (KLS) cells (n = 3 young, n = 3 old). Error bars represent SEM.
We next examined the expression pattern of Rantes in aged versus young BM cells. Rantes is known to be secreted from stromal cells (osteoblasts), monocytes and CD8+ T cells.27,28 We were particularly interested in the bone-adherent fraction, collectively termed here the “bone-derived stroma,“ because these cells are a key component of the HSC niche. Bone-derived stromal cells (see “Quantitative real-time PCR”) were extracted from old or young mice, and RNA was isolated. Expression of Rantes, measured by quantitative real-time (qrt) PCR, was increased by 6-fold in old bone-derived stromal cells compared with the young stromal cells (Figure 2C). Sorted lineage-positive (lin+) cells from old BM also demonstrated similar expression levels relative to young BM cells. However, in hematopoietic stem/progenitor cells (KLS; c-Kit+, Lin−, Sca-1+), Rantes expression was not different (Figure 2C). This suggests that Rantes is increased in the HSC niche via stromal cell secretion as well as from local HSC progeny. This is consistent with recent findings that aged thymus cells also express more Rantes.29
Rantes overexpression causes a decrease in T lymphocytes and a decrease in prolymphoid transcription factors
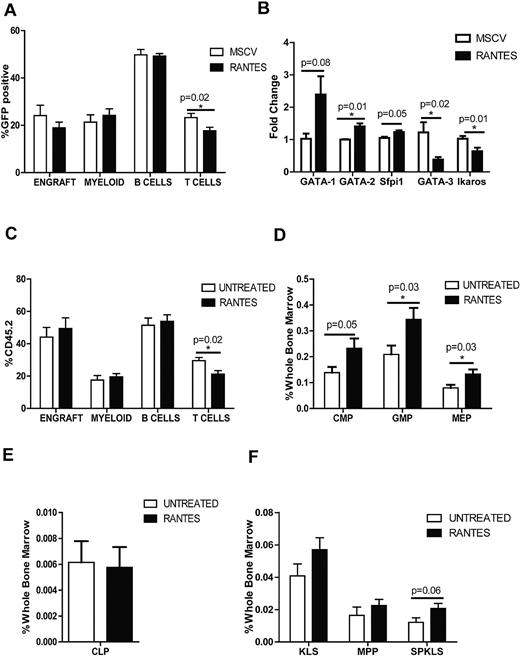
We next asked whether we could mimic the “aged” cellular environment by overexpressing Rantes in the BM. We isolated BM progenitor cells from CD45.2 mice and transduced them with MSCV-based vector expressing either Rantes-IRES-GFP or GFP-only as a control. The cells were then transplanted into CD45.1 mice, and the PB was analyzed every 4 weeks. To compare the control versus Rantes-overexpressing mice, we analyzed the percentage of donor-derived GFP+ cells for myeloid, B- and T-cell compartments. We observed a significant decrease in T cells (23.3% ± 1.7% vs 17.7% ± 1.5%) in PB of Rantes-overexpressing mice compared to control mice at 12 weeks (Figure 3A, supplemental Figure 2A). At 16 weeks, Rantes overexpression resulted in a slight increase of myeloid cells (supplemental Figure 2B). Probably because of a wider variation in the WT group, T-cell output was not significantly different at 16th week (22.5% ± 2.7 vs 17.9% ± 1.5%; supplemental Figure 2A). The shift toward production of myeloid cells became more pronounced at a later time point (24.4% ± 3.2% vs 33.2% ± 3.7%; supplemental Figure 2A) which suggests that in combination with other aging factors, Rantes drives a myeloid-skewing phenotype.
Exposure to Rantes causes myeloid skewing. (A) The graph shows the proportions of lineages (Myeloid, B cells, and T cells) of the GFP+ donor-derived WBCs transduced with MSCV-GFP or MSCV-Rantes-IRES-GFP analyzed 12 weeks after transplantation (n = 10 MSCV, n = 10 Rantes). Overall engraftment of transduced cells is also indicated (“Engraft”). (B) Quantitative real-time PCR analysis of the indicated genes in progenitors (LSGFP+ cells) from MSCV-GFP or Rantes-GFP transduced progenitors after 48 hours in vitro (n = 3 MSCV, n = 3 Rantes). (C) PB analyses of mice transplanted with HSCs that are treated or untreated with Rantes ex vivo before competitive transplantation. Donor contribution to myeloid and lymphoid lineages is presented as the percentage of donor-derived myeloid, B, and T cells 16 weeks posttransplantation. (D) BM frequencies of donor-derived myeloid progenitors in BM from mice transplanted with Rantes- or untreated HSCs (SPKLS) in a competitive transplantation: CMPs, GMPs, MEPs. Analysis of HSC-derived progeny in recipients 5 months posttransplantation (n = 7 Untreated, n = 7 Rantes). (E) BM frequencies of donor-derived CLPs from Rantes- or untreated HSC as in panel D. (F) BM frequencies of donor-derived stem and progenitor populations: KLS, MPP (KLS Flk2+), SPKLS, SPKLS-CD150+ after transplantation of Rantes- or untreated HSC as in panel D. CMP: Lin−, IL7rα−, Sca-1−, c-Kit+, FcγRloCD34+; GMP: Lin−, IL7rα−, Sca-1−, c-Kit+, FcγR+CD34+; MEP: Lin−, IL7rα−, Sca-1−, c-Kit+, FcγR−CD34−; and CLP: Lin−, IL7rα+, Sca-1lo, c-Kitlo, Flt3+ (n = 7 Untreated, n = 7 Rantes). Error bars represent SEM. Representative of 2 experiments
Exposure to Rantes causes myeloid skewing. (A) The graph shows the proportions of lineages (Myeloid, B cells, and T cells) of the GFP+ donor-derived WBCs transduced with MSCV-GFP or MSCV-Rantes-IRES-GFP analyzed 12 weeks after transplantation (n = 10 MSCV, n = 10 Rantes). Overall engraftment of transduced cells is also indicated (“Engraft”). (B) Quantitative real-time PCR analysis of the indicated genes in progenitors (LSGFP+ cells) from MSCV-GFP or Rantes-GFP transduced progenitors after 48 hours in vitro (n = 3 MSCV, n = 3 Rantes). (C) PB analyses of mice transplanted with HSCs that are treated or untreated with Rantes ex vivo before competitive transplantation. Donor contribution to myeloid and lymphoid lineages is presented as the percentage of donor-derived myeloid, B, and T cells 16 weeks posttransplantation. (D) BM frequencies of donor-derived myeloid progenitors in BM from mice transplanted with Rantes- or untreated HSCs (SPKLS) in a competitive transplantation: CMPs, GMPs, MEPs. Analysis of HSC-derived progeny in recipients 5 months posttransplantation (n = 7 Untreated, n = 7 Rantes). (E) BM frequencies of donor-derived CLPs from Rantes- or untreated HSC as in panel D. (F) BM frequencies of donor-derived stem and progenitor populations: KLS, MPP (KLS Flk2+), SPKLS, SPKLS-CD150+ after transplantation of Rantes- or untreated HSC as in panel D. CMP: Lin−, IL7rα−, Sca-1−, c-Kit+, FcγRloCD34+; GMP: Lin−, IL7rα−, Sca-1−, c-Kit+, FcγR+CD34+; MEP: Lin−, IL7rα−, Sca-1−, c-Kit+, FcγR−CD34−; and CLP: Lin−, IL7rα+, Sca-1lo, c-Kitlo, Flt3+ (n = 7 Untreated, n = 7 Rantes). Error bars represent SEM. Representative of 2 experiments
Next, we investigated whether overexpression of Rantes resulted in gene expression changes in BM progenitors. Progenitors were transduced and cultured for 48 hours, and GFP+ progenitors were sorted for RNA isolation. Expression of key transcription factors such as Gata1, Gata2, Sfpi1 (which are known regulatos of myeloid differentiation) and Gata3, Ikaros (which are T-cell specification factors were measured by qrt-PCR), revealing that expression of Gata1, Gata2 and Sfpi1 increased (2.3-, 1.4-, and 1.2-fold, respectively) whereas Gata3 and Ikaros levels were decreased (40% and 60% lower, respectively) in Rantes-overexpressing cells (Figure 3B). Gata2, Gata3, and Ikaros levels were slightly but significantly different in Rantes-overexpressing progenitors (Figure 3B). Thus, Rantes exposure can induce expression of promyeloid transcription factors and reduce expression of prolymphoid factors.30-33
Ex vivo treatment of HSCs with Rantes before transplantation results in T-cell deficiency and expands myeloid progenitors
We considered whether the effect we observed by overexpression could be recapitulated with a brief (only 4 hours) ex vivo exposure of stem cells to Rantes, followed by transplantation. We isolated HSCs (SPKLS) from CD45.2 mice and treated cells with 30 ng/mL recombinant Rantes protein for 4 hours before transplanting 300 HSCs into CD45.1 mice together with 2 × 105 CD45.1 competitor whole BM cells. PB analyzed every 4 weeks posttransplantation to determine engraftment of donor-derived cells and their differentiation behavior. We observed the same lineage-skewing phenotype as when Rantes was overexpressed, with significantly lower T-cell numbers 16 weeks after transplantation (29.6% ± 1.9% vs 21.2% ± 2.2%; Figure 3C, supplemental Figure 3A). Thus, even brief exposure of stem cells to Rantes has a significant impact on their differentiation propensity.
To determine whether the decrease in the T-cell output of donor-derived stem cells in the PB was a reflection of bias in hematopoietic stem and progenitors, recipient mice were killed 20–22 weeks after transplantation, and donor-derived stem and progenitor cell compartments were examined. When donor cells were treated with Rantes before transplantation, we observed a significant increase in donor-derived early committed progenitors of CMPs (0.14% ± 0.02% vs 0.23% ± 0.04%), MEPs (0.08% ± 0.01% vs 0.13% ± 0.02%), and GMPs (0.20% ± 0.04% vs 0.34% ± 0.03%; Figure 3D, supplemental Figure 3B), but no differences in CLPs (Figure 3E, supplemental Figure 4). Within the stem cell compartment, HSCs were slightly increased in the Rantes-treated group (0.012% ± 0.003% vs 0.021% ± 0.003%; Figure 3F). We detected increased production of myeloid progenitors in the Rantes-treated group after we observed a T-cell defect in the PB analysis. This suggests that lineage skewing toward myelopoiesis occur at later stage, at the expense of T cells first. Therefore, this shift toward increased myelopoiesis is associated with increased production of myeloid progenitors and long-term HSCs after Rantes exposure, and is highly correlated with the functional changes that occur with normal HSC aging.
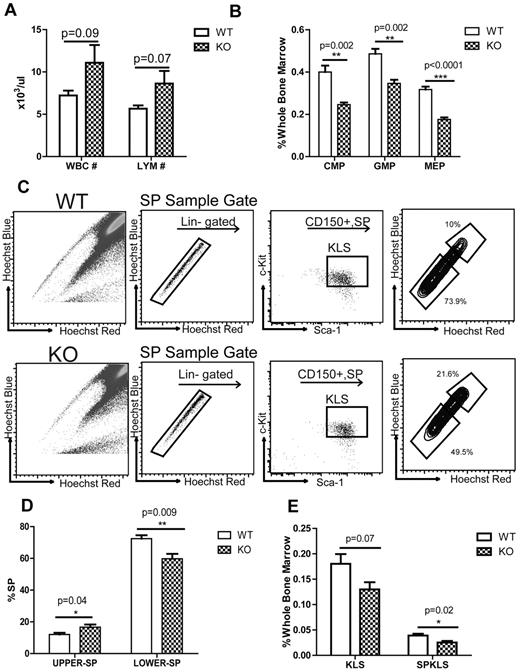
Mid-age Rantes KO mice have increased numbers of lymphocytes and reduced numbers of myeloid progenitors
We have shown above that after in vitro or in vivo exposure to Rantes, hematopoietic differentiation is biased against T-cell development. We thus considered whether elimination of Rantes would have the opposite effect, namely enhancement of lymphoid development. To investigate this, we obtained Rantes KO mice. We examined 5- to 6-month-old KO animals for lineage development bias and differences in their myeloid and lymphoid progenitors. Complete blood count (CBC) analysis of 5- to 6-month-old mice revealed that KO mice had a higher numbers of white blood cell (WBC; 7.2 × 103 ± 0.6 × 103/μL vs 11.1 × 103 ± 2.1 × 103/μL) and lymphocyte cell counts (Lym; 5.6 × 103 ± 0.4 × 103/μL vs 8.6 × 103 ± 1.5 × 103/μL) compared with the wild-type mice (Figure 4A). We also observed significant reduction in both frequencies and absolute numbers of CMPs (0.4% ± 0.03% vs 0.25% ± 0.01%), GMPs (0.49% ± 0.02% vs 0.35%% ± 0.02%), and MEPs (0.32% ± 0.01% vs 0.18% ± 0.01%) in the BM of Rantes KO mice (Figure 4B, supplemental Figure 5A), but no change in the proportion and absolute numbers of CLPs (supplemental Figure 5B-C). Thus, Rantes deficiency results in a relative expansion of lymphocytes in the PB together with a decrease in myeloid progenitors in the BM, demonstrating the impact of Rantes on the balance of the lymphoid versus myeloid development.
Rantes KO mice have less myeloid progenitors and less myeloid-biased stem cells. (A) CBC analyses of 5- to 6-month-old WT and Rantes KO mice. Graphical representation of CBC analysis of WT or KO mice. (B) BM frequencies of myeloid progenitors (CMP, GMP, MEP) from 5- to 6-month-old WT and KO mice. (C) BM derived from WT or Rantes KO mice was isolated and analyzed for Ly- and My-bi HSCs as follows: KLS cells were identified, followed by the CD150+ fraction, followed by analysis of lower and upper SP phenotypes. Representative FACS profiles of lower and upper SPKLS-CD150+ HSCs from WT and Rantes KO mice. (D) Frequencies of lower and upper SPKLS-CD150+ cells from WT and KO mice. (E) BM frequencies of stem and progenitor populations: KSL, SPKLS from 5- to 6-month-old WT and KO mice. n = 5 WT, n = 5 KO in (A-D). Error bars represent SEM. Representative of 2 experiments
Rantes KO mice have less myeloid progenitors and less myeloid-biased stem cells. (A) CBC analyses of 5- to 6-month-old WT and Rantes KO mice. Graphical representation of CBC analysis of WT or KO mice. (B) BM frequencies of myeloid progenitors (CMP, GMP, MEP) from 5- to 6-month-old WT and KO mice. (C) BM derived from WT or Rantes KO mice was isolated and analyzed for Ly- and My-bi HSCs as follows: KLS cells were identified, followed by the CD150+ fraction, followed by analysis of lower and upper SP phenotypes. Representative FACS profiles of lower and upper SPKLS-CD150+ HSCs from WT and Rantes KO mice. (D) Frequencies of lower and upper SPKLS-CD150+ cells from WT and KO mice. (E) BM frequencies of stem and progenitor populations: KSL, SPKLS from 5- to 6-month-old WT and KO mice. n = 5 WT, n = 5 KO in (A-D). Error bars represent SEM. Representative of 2 experiments
Myeloid-biased stem cells are reduced in the BM of Rantes KO mice
We next investigated whether HSC number and/or subtypes were altered in the KO animals. We again first selected KLS-CD150+ cells to identify the longest-term most conservatively defined HSCs, and then analyzed the upper and lower SP (lymphoid- and myeloid-biased populations, respectively) in 5- to 6-month-old KO mice. From this analysis, we found a significantly higher percentage of upper SPKLS-CD150+ (12% ± 1% vs 16.7% ± 1.7%) whereas the lower SPKLS-CD150+ percentage was found to be decreased significantly (72.5% ± 2.1% vs 59.8% ± 3%) in the KO BM (Figure 4C-D). We also observed a significant reduction in SPKLS (0.039% ± 0.004% vs 0.025% ± 0.003%) and KLS cells (0.18% ± 0.019% vs 0.13% ± 0.014%) which have been shown to increase in the aged mice7 (Figure 4E). We also calculated absolute numbers of stem cells and found a significant reduction in both KLS and SPKLS populations (supplemental Figure 5C).
PB, committed progenitor, and stem cell analysis revealed that Rantes KO mice have more lymphocytes and more Ly-bi HSCs, as well as fewer myeloid progenitors, and My-bi HSCs. Together, this constellation of features is the opposite of what is found with normal HSC aging, suggesting that elimination of Rantes results in functionally and phenotypically younger HSCs.
To begin to examine the possible mechanisms for the impact of Rantes loss on HSC function, we re-examined Gata2, Gata3, and Ikaros expression. We found that expression of Gata2, a known promyeloid transcription factor, was 40% lower in Rantes KO HSCs, whereas expression of Gata3 and Ikaros, which are known to regulate lymphoid differentiation in HSCs, was increased significantly (2-fold and 6-fold). Here we provided evidence that Rantes may act partly via regulation of Gata2, Gata3, and Ikaros which affects HSC maintenance and differentiation (supplemental Figure 6).30,32,33
KO HSCs exhibit similar engraftment potential and generate more lymphoid-biased stem cells in transplantations
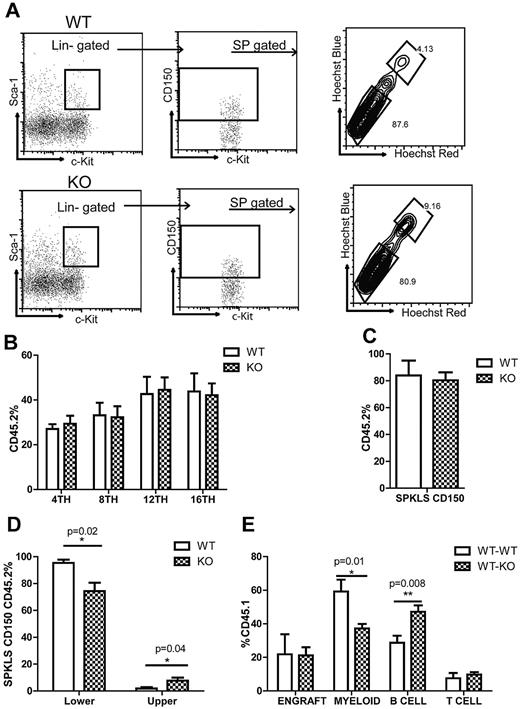
We next asked whether KO stem cells exhibit the same phenotype of fewer My-bi stem cells and more Ly-bi stem cells in a transplantation setting. We isolated HSCs (SPKLS-CD150+) from 9-month-old WT or KO mice and transplanted 300 HSCs into CD45.1 mice together with 2 × 105 CD45.1 competitor whole BM cells. We also analyzed BM of 9-month-old KO and WT mice before transplantation for HSC subtypes and saw that the percentage of My-bi stem cells was lower (80.9% vs 87.6%) whereas the percentage of Ly-bi stem cells was higher (9.2% vs 4.1%) in KO mice (Figure 5A). PB was analyzed every 4 weeks posttransplantation to determine engraftment of donor-derived cells and their differentiation behavior. We did not detect any change in the overall engraftment of KO cells in PB (Figure 5B) but we observed a slight increase in T cells 8 weeks after transplantation (data not shown) but no change after that.
Rantes KO HSCs exhibit similar engraftment potential. (A) BM derived from 9-month-old WT or Rantes KO mice was isolated and analyzed for Ly- and My-bi HSCs as follows: KLS cells were identified, followed by the CD150+ fraction, followed by analysis of lower and upper SP phenotypes. Representative FACS profiles of lower and upper SPKLS-CD150+ HSCs from WT and Rantes KO mice. (B) Overall engraftment of 9-month-old WT or KO HSCs (SPKLS-CD150+) in the PB of recipient mice at 4th, 8th, 12th, and 16th week after transplantation (n = 5 WT, n = 6 KO). (C) Analysis of the BM of recipient mice to determine donor cell contribution to the total stem cell compartment (SPKLS-CD150+) 20 weeks posttransplantation of WT or KO HSCs. (D) Analysis of the BM of recipient mice for the frequencies of donor-derived lower and upper SPKLS-CD150+ cells. (E) Transplantation of WT HSCs from 18-month-old mice into WT or Rantes KO mice. The graph shows the lineage distribution of donor-derived cells in the WT or KO mice at 12 weeks posttranplantation. Overall engraftment of donor cells is also indicated as (“Engraft”). Error bars represent SEM. Representative of 2 experiments (n = 6 WT, n = 7 KO).
Rantes KO HSCs exhibit similar engraftment potential. (A) BM derived from 9-month-old WT or Rantes KO mice was isolated and analyzed for Ly- and My-bi HSCs as follows: KLS cells were identified, followed by the CD150+ fraction, followed by analysis of lower and upper SP phenotypes. Representative FACS profiles of lower and upper SPKLS-CD150+ HSCs from WT and Rantes KO mice. (B) Overall engraftment of 9-month-old WT or KO HSCs (SPKLS-CD150+) in the PB of recipient mice at 4th, 8th, 12th, and 16th week after transplantation (n = 5 WT, n = 6 KO). (C) Analysis of the BM of recipient mice to determine donor cell contribution to the total stem cell compartment (SPKLS-CD150+) 20 weeks posttransplantation of WT or KO HSCs. (D) Analysis of the BM of recipient mice for the frequencies of donor-derived lower and upper SPKLS-CD150+ cells. (E) Transplantation of WT HSCs from 18-month-old mice into WT or Rantes KO mice. The graph shows the lineage distribution of donor-derived cells in the WT or KO mice at 12 weeks posttranplantation. Overall engraftment of donor cells is also indicated as (“Engraft”). Error bars represent SEM. Representative of 2 experiments (n = 6 WT, n = 7 KO).
We then analyzed BM of recipient mice 5 months after transplantation. We found that within the total stem cell compartment (SPKLS-CD150+) the percentage of donor-derived HSCs (SPKLS-CD150+ 45.2+) was the same between WT and KO groups (Figure 5C). But when we compared lymphoid and myeloid-biased HSCs within the donor-derived cells, the percentage of KO-derived My-bi HSCs was significantly lower than WT (74.3% ± 6.2% vs 95.4% ± 2.4%) whereas the percentage of KO-derived Ly-bi HSCs was significantly higher (7.7% ± 2.2 vs 1.9% ± 1%; Figure 5D).
Next, we asked whether the KO environment could have an effect on stem cell lineage choice, because Rantes is a secreted factor. We transplanted 17- to 18-month-old WT HSCs (SPKLS CD150+) into either 2- to 3-month-old WT or KO mice. We compared the lineage distribution of old HSCs within WT or KO mice and found that old HSCs generated significantly less myeloid cells (59.2% ± 7.1% vs 37.2% ± 2.6%) when transplanted into KO mice 12 weeks after transplantation (Figure 5E). They also gave rise to significantly more B cells (28.7% ± 4.1% vs 47.2% ± 3.7%; Figure 5E). These results suggested that old HSCs in a Rantes-deficient environment could generate fewer myeloid cells, consistent with an amelioration of the pro-aging environment.
Rantes KO progenitors cells have decreased mTOR activity
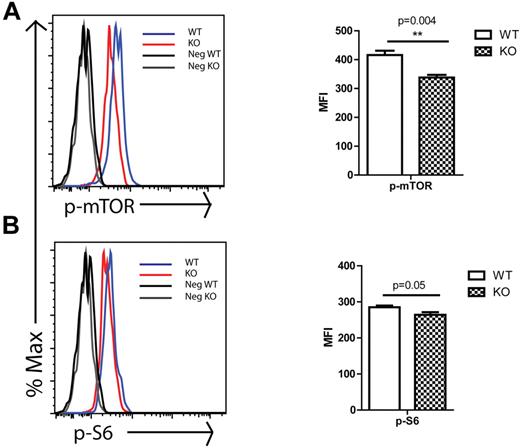
We next considered possible molecular mechanisms that would contribute to the observed Rantes KO phenotype. It has been shown that Rantes activates mTOR pathway in T cells which leads to initiation of protein translation by phosphorylating and deactivating the translation inhibitor 4E-BP1, which results in its dissociation from the eukaryotic initiating factor-4E (Eif-4E).34 Because the mTOR pathway was implicated in aging and has been reported to be increased in aged hematopoietic stem and progenitors,35 we decided to analyze mTOR activity in Rantes KO stem/progenitors. We isolated BM cells from 2-month-old WT or KO mice and analyzed them for the level of p-mTOR which is the active form of the protein and its down-stream target phospho-ribosomal protein S6 (p-S6) via intracellular staining of progenitors (KLS). Analysis revealed that p-mTOR levels were significantly decreased in KO KLS cells (Figure 6A). Consistent with the p-mTOR levels, the levels of p-S6, one of the downstream targets of mTOR complex 1 (mTORC1) through S6K, were also significantly reduced (Figure 6B). Although these changes are relatively small, ribosomal protein S6 is one of the downstream components of mTOR, likely contributing to a larger overall effect. These data indicate that Rantes acts at least partly through the mTOR pathway, likely contributing to the younger HSC phenotype in Rantes KO animals. It has been reported that activation of mTOR pathway through conditional deletion of tuberous sclerosis complex 1 (Tsc1) protein in HSCs causes a premature aging phenotype, whereas inhibition of mTOR pathway through rapamaycin, an mTOR inhibitor, treatment results in extension of lifespan and restores the self-renewal and reconstitution potential of aged HSCs.35,36 Thus, our results were consistent with previous studies indicating that lower mTOR activity leads to a younger HSC phenotype.
mTOR activity in WT and KO progenitors. BM cells were isolated from 2-month-old WT or KO mice and stained with Abs specific to cell-surface markers to identify c-Kit+lin−Sca-1+ (KLS) progenitors, followed by intracellular staining with Abs against (A) p-mTOR and (B) p-S6. (Left side) Histograms of progenitors (KLS) that are representative of 3 independent experiments each involving 3 to 5 mice in each group. Gray and black lines are negative controls (secondary Ab only for panels A and B), black line for cells from WT mice and gray line for cells from KO mice. Blue line represents profile of stained BM cells from WT mice and red line represents profile of stained BM cells from KO mice. The median fluorescence intensity (MFI) ± SD of representative of one experiment is shown at the right side. **P < .01; *P < .05 (n = 4 WT and n = 4 KO).
mTOR activity in WT and KO progenitors. BM cells were isolated from 2-month-old WT or KO mice and stained with Abs specific to cell-surface markers to identify c-Kit+lin−Sca-1+ (KLS) progenitors, followed by intracellular staining with Abs against (A) p-mTOR and (B) p-S6. (Left side) Histograms of progenitors (KLS) that are representative of 3 independent experiments each involving 3 to 5 mice in each group. Gray and black lines are negative controls (secondary Ab only for panels A and B), black line for cells from WT mice and gray line for cells from KO mice. Blue line represents profile of stained BM cells from WT mice and red line represents profile of stained BM cells from KO mice. The median fluorescence intensity (MFI) ± SD of representative of one experiment is shown at the right side. **P < .01; *P < .05 (n = 4 WT and n = 4 KO).
Discussion
Previous studies on the effects of aging on HSCs demonstrated that aged HSCs are not as potent as their younger counterparts, and they exhibit marked myeloid differentiation bias.3-5,10 Recently, multiple groups reported that the HSC pool consists of multiple HSC subtypes with distinct properties.14,15,17 These subtypes change in their proportions with age, such that My-bi HSCs become much more prevalent.16,17 In the current study, we show that niche factors secreted from aging stromal cells play a critical role in this HSC subtype shift, and identify Rantes as a key proinflammatory cytokine which accumulates in aged BM and is expressed by stromal as well as differentiated blood cells.26
Our data draw a striking parallel with recent findings on the affects of aging on neurogenesis. Through heterochronic parabiosis, an aging systemic milieu has been shown to negatively regulate neural functions and adult neurogenesis in mice.20 By surveying secreted proteins in the circulating plasma using a similar approach to ours in the BM, a small set of chemokines was found to be elevated, including Ccl11. Injection of Ccl11 into young mice induced impaired learning and memory and reduced neurogenesis, demonstrating a key role. It is striking that this study also found similar immune modulators. It is possible that a more extensive survey of cytokines and signaling molecules on aged BM would reveal additional contributing factors.
Rantes, like Ccl11, is known to act as a chemoattractant for differentiated cells such as monocytes, eosinophils, T, NK, and dendritic cells.26 It is released from stromal cells in damaged tissue, binds to glycosaminoglycans on the endothelium, and attracts immune cells from the PB to sites of inflammation. Activated T cells express Rantes within 3 to 5 days after stimulation, hence amplifying the immune response. Rantes binds and activates several G-protein coupled receptors (GPCR), namely Ccr-1, -3, and -5. Ccr5 is the major Rantes receptor and it also acts as a coreceptor for HIV uptake.26 It has been reported that Ccr5 can form heterodimers with Ccr-2, which is the closest homolog of Ccr-5, and can also form heterooligomers together with Cxcr4 and Ccr2.37 Rantes can also bind to other receptors such as Cd44, and may also act through a GPCR-independent pathway.38 Thus, there are multiple possible routes of action of Rantes on HSCs.
Strikingly, Ccl11 also uses the receptors Ccr-2, -3, and -5.39 Thus, both Rantes and Ccl11, independently shown in 2 different systems to have a negative impact on stem cell aging, act via the same receptors, supporting the concept that these proinflammatory chemokines play a role in aging. Our chemokine bead array did not test for Ccl11 changes in BM serum, so this could have been missed. Rantes was not observed to increase in the peripheral blood in the neurogenesis study, a difference that could be attributed to the source of the test material (PB serum vs BM serum) or other technical factors. It is not clear what receptors are mediating the effects; however, we do note that in our previous study we found that Ccr-2 mRNA expression levels increased significantly with aging.7 Thus, it is possible that Rantes can bind to Ccr-2 or other receptors such as Cd44 on the HSCs and activate downstream targets directly in HSCs.
Here, we show for the first time that an inflammatory cytokine, Rantes, in the BM of mice can contribute to the lineage skewing phenotype in the PB on overexpression or brief ex vivo exposure of HSCs. Exposing the cells to Rantes appears to have a direct effect on the fate choices of both stem and progenitor cells; hence, we may be mimicking the aged environment and promoting lineage skewing with Rantes overexpression. Enforced expression of Rantes induces expression of promyeloid transcription factors including Gata-2 which is important for the maintenance and expansion of multipotent progenitors and HSCs40 and reduces expression of lymphoid-specification genes in the progenitors, such as Ikaros and Gata3 which is shown to regulate early lymphopoiesis and T-cell development.30,32 We speculate that exposing stem and progenitor cells to Rantes directs them toward myeloid differentiation at the expense of lymphoid differentiation by shutting down the expression of lymphoid-specification genes and inducing myeloid specification genes. Together with Rantes, we also identified Mig (Cxcl9) in our CBA assay. Preliminary experiments with Mig did not reveal a significant effect, but perhaps in combination with Rantes it contributes to the aging phenotype.
In contrast, in Rantes KO mice, the percentage of myeloid progenitors and My-bi HSCs was decreased. Concomitantly, we observed an expansion of lymphocytes in the periphery and Ly-bi stem cells in the BM of Rantes KO mice. Elimination of Rantes may render the environment more favorable for Ly-bi stem cells, which could then give rise to more lymphocyte progeny. These data are consistent with our heterochronic transplantations, which show less myeloid progeny after transplantation of old HSCs into a young environment. The young environment (via Rantes and possibly other cytokines) may allow maintenance of HSC heterogeneity, allowing efficient generation of both Ly-bi and My-bi stem cells in balance, hence generating less myeloid cells from old cells. In contrast, the old environment favors My-bi stem cells which results in generation of more myeloid progeny. Rantes may act at least in part via Gata2, which could also account for the reduced numbers of MEPs in the KO mice because it regulates early erythrocyte development.41 Through Gata3 and Ikaros, it may regulate lymphopoiesis and T-cell development causing an increase in Ly-bi HSCs and T lymphocytes.30,32 In our previous report, we found that Ikaros is 1 of the genes differentially expressed between upper and lower SPKLS cells with a higher expression in the Ly-bi upper SPKLS cells which further supports that KO animals have increased numbers of Ly-bi HSCs.17
We also tested whether KO HSCs still exhibit the same My- and Ly-bi HSC subtype difference after transplantation and found that they continue to produce more Ly- and less My-bi HSCs. Although we did not detect any change in their differentiation potential or engraftment, it is possible that other factors present in the old BM might be required for full manifestation of the myeloid skewing phenotype. Alternatively, because Rantes is a secreted protein, KO HSCs placed into a WT environment might not fully exhibit the younger differentiation phenotype if Rantes continues to be expressed from remaining WT cells. To address this possibility, we also used Rantes KO mice as a recipient, and transplanted aged WT HSCs into a WT or KO environment. In this case, we saw a significant decrease in myeloid progeny in the Rantes-deficient environment. This is consistent with our hypothesis that in a Rantes-deficient microenvironment, old HSCs will be less biased toward myelopoiesis at the expense of lymphopoiesis. In future work, it will be important to determine the major source of Rantes exposure for the HSCs. Rantes can be secreted by stromal or endothelial cells, as well as differentiated hematopoietic progeny. The partial reduction in HSC differentiation bias when transplanted into Rantes KO mice suggests that Rantes from stromal elements may play a significant role.
A recent report demonstrated that in T cells, Rantes causes phosphorylation of mTOR and its downstream target proteins.34 Analysis of mTOR activity in Rantes-null hematopoietic stem cell/progenitors (KLS) revealed that they had significantly lower p-mTOR and p-S6 which is the down-stream target of mTOR compex-1 (mTORC1). mTOR pathway links energy and nutrient sensing to growth and division. mTOR acts as a catalytic subunit of 2 distinct protein complexes called mTORC1 and mTORC2 which are distinguished by the presence of unique accessory proteins. Rapamycin inhibits mTORC1 but not mTORC2. Because of its role in growth and nutrition sensing, mTOR could be seen as one of the master regulator of aging. It has been reported that dietary restriction which is the only natural way to slow down the aging process acts trough inhibition of mTORC1 which was further supported through genetic studies like S6K1 deficiency in mice results in a lifespan extension and changes in gene expression which is similar to dietary restriction.42 It has been also reported that in HSCs, constitutive mTOR activation through conditional deletion of TSC1 causes up-regulation of p16, p19, and p21 gene expression which leads to depletion of HSCs. Moreover, they observed an increased mTOR activity in aged HSCs; inhibition of mTOR activity through rapamycin treatment of aged mice partly rejuvenated the HSC pool.35 In another study, PI3K pathway activation through Pten deletion also resulted in hyperproliferation and depletion of HSCs, and through rapamycin treatment, reconstitution potential of Pten-deficient HSCs was restored.43 All these studies indicate that mTOR inhibition is required to preserve HSC quality. In our study, we also detected a decrease in mTOR activity in Rantes KO mice which might sustain the HSCs in a younger state and maintain the decreased numbers of My-bi HSCs and increased numbers of Ly-bi HSCs.
This study reveals that the balance between My- and Ly-bi HSCs, and the change in that balance with age, is regulated at least in part by Rantes. Better understanding of the aging niche and niche-associated factors could help ameliorate some of the hematopoietic effects of the aging process, possibly reducing myeloid disorders and improving the immune response in the elderly.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Allison Rosen and Yayun Zheng for technical support.
This work was supported by the Ellison Medical Foundation and National Institutes of Health grants AG034451, 1RC2AG036562 and DK092883.
National Institutes of Health
Authorship
Contribution: A.V.E. designed and performed experiments, analyzed and interpreted data, and wrote the manuscript; N.C.B. designed and performed experiments, and analyzed and interpreted data; and M.A.G. directed the study, contributed to experimental design and interpretation, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Margaret A. Goodell, Baylor College of Medicine, One Baylor Plaza, N1030, Houston, TX 77030; e-mail: goodell@bcm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal