Abstract
Enteropathy-associated T-cell lymphoma (EATL) is a complication of celiac disease (CD). This tumor derives from the neoplastic transformation of aberrant intraepithelial T lymphocytes emerging in celiac patients unresponsive to a gluten-free diet. Poor adherence to a gluten-free diet, HLA-DQ2 homozygosity, and late diagnosis of CD are recognized as risk factors for malignant evolution of CD. Recurrence of diarrhea, unexplained weight loss, abdominal pain, fever, and night sweating should alert physicians to this complication. The suspicion of EATL should lead to an extensive diagnostic workup in which magnetic resonance enteroclysis, positron emission tomography scan, and histologic identification of lesions represent the best options. Treatment includes high-dose chemotherapy preceded by surgical resection and followed by autologous stem cell transplantation, although biologic therapies seem to be promising. Strict adherence to a gluten-free diet remains the only way to prevent EATL.
Introduction
Celiac disease (CD) is a chronic gluten-sensitive enteropathy characterized by a high prevalence in the general population and an increased mortality.1,2 It is well known that the increased mortality is mainly the result of the complications of CD itself, represented by refractory CD (RCD) and enteropathy-associated T-cell lymphoma (EATL).2
RCD is a form of CD that does not respond histologically to at least 12 months of a strict gluten-free diet (GFD).3 RCD can also evolve in patients who initially responded normally to a GFD and who are still maintaining a strict GFD. On the basis of the intraepithelial lymphocyte (IEL) population, RCD is further classified into type 1 and type 2. RCD type 1 (RCD1) is characterized by persisting villous atrophy despite a strict GFD associated with increased but still phenotypically normal IELs. Conversely, a clonal expansion of abnormal IELs lacking surface CD3, CD8, and T-cell receptor (TCR) markers but expressing intracellular CD3 indicates RCD type 2 (RCD2), a condition that frequently evolves into EATL, the most serious complication of CD.4
In the past, up to 20% of celiac patients were considered to be affected by a nonresponsive form of CD. This was obviously because of the lack of consensus on the definition of nonresponsiveness.4 In the last few years, some evidence has suggested that the prevalence of RCD is low, ranging from 0.6% to 1.5%.3,5,6 Although the presence of RCD in CD is probably to be approximately 1%, the prevalence of RCD2 among patients with RCD is far from being ascertained. Probably because of not only the great differences in the diagnostic criteria,4 but also referral bias, different papers report very different results, which vary from 15% to 75%.7-9 Finally, although the incidence of EATL was reported to be rare in the general population (1 per million person-years),10,11 it was shown that it occurs in 60% to 80% of patients with RCD2 within 5 years.7-9,12 The description of EATL arising in patients with RCD1 seems to be exceptional.7 Recent evidence suggests that non-EATLs, including intestinal B-cell and extraintestinal T-cell lymphomas, may rarely occur in celiac patients.13,14
As far as the prognosis of all these conditions is concerned, there are no doubts that it is much worse than that of uncomplicated CD.2,15 The 5-year survival rate is reported to be between 80% and 96% in patients with RCD1, but it is only between 40% and 58% in patients with RCD2.6-8,15 Five-year survival dropped to between 8% and 20% in RCD2 patients who developed EATL.8,9,16
Natural history
The first paper suggesting that CD predisposes to intestinal lymphoma was published 50 years ago,17 and 5 years ago it was shown that EATL is strongly linked to CD.18 In the last few years, it has emerged that several factors increase the risk of developing malignant complications in patients with CD. This is the case for not only poor adherence to a GFD but also advanced age at diagnosis (> 50 years), diagnostic delay for CD (> 10 years), and HLA-DQ2 homozygosity.2,19 Moreover, we have recently hypothesized that the total amount of gluten consumed not only after but also before the start of a GFD could play a role in inducing celiac complications.2 It should, however, be noted that most of the evidence supporting a protective effect of a GFD is indirect and that studies directly testing this hypothesis are scarce. Hence, it follows that this is an area that needs further study with regard to subgroups of CD patients and subgroups of lymphoma.
Although some celiac patients complicating directly into EATL have been described,12 most of the time celiac complications arise as either RCD1 or RCD2. Some patients can also develop ulcerative jejunoileitis (UJI), a premalignant small bowel disorder that shares many immunopathologic features with RCD2 and is characterized by multiple ulcerations evolving in strictures of the intestinal wall.20,21 Both RCD2 and UJI can subsequently degenerate into EATL20,21 through a progressive accumulation in the intestinal epithelium of aberrant (CD3ϵ+, CD103+, CD8−, CD4−, TCR-αβ−) and clonal (restricted rearrangements of TCR-γ chain) IELs,22 abnormally expanded by the antiapoptotic action of IL-15 (Figure 1).23,24
Schematic representation of IL-15 lymphomagenic action leading to the emergence of EATL in CD. In active CD mucosa, IL-15, mainly produced by epithelial cells and dendritic cells (DCs), which present tissue transglutaminase (tTG)–deamidated gluten peptides to T cells in the context of HLA-DQ2 or HLA-DQ8 molecules, promotes the IEL cytotoxicity against epithelial cells by activating the perforin/granzyme system and favoring the interaction between the homodimeric NK activating receptor NFG2D and the major histocompatibility complex class I–related ligands (MIC), thus leading to enterocyte apoptosis. In addition, IL-15 inhibits IEL apoptosis, thus favoring the emergence of neoplastic IEL clonal proliferations, leading to the development of EATL. Activated gluten-reactive T helper cell type 1 cells (Th1) produce high levels of the proinflammatory cytokine IFN-γ, which contributes to the mucosal damage by further activating the cytotoxicity of IELs against enterocytes.
Schematic representation of IL-15 lymphomagenic action leading to the emergence of EATL in CD. In active CD mucosa, IL-15, mainly produced by epithelial cells and dendritic cells (DCs), which present tissue transglutaminase (tTG)–deamidated gluten peptides to T cells in the context of HLA-DQ2 or HLA-DQ8 molecules, promotes the IEL cytotoxicity against epithelial cells by activating the perforin/granzyme system and favoring the interaction between the homodimeric NK activating receptor NFG2D and the major histocompatibility complex class I–related ligands (MIC), thus leading to enterocyte apoptosis. In addition, IL-15 inhibits IEL apoptosis, thus favoring the emergence of neoplastic IEL clonal proliferations, leading to the development of EATL. Activated gluten-reactive T helper cell type 1 cells (Th1) produce high levels of the proinflammatory cytokine IFN-γ, which contributes to the mucosal damage by further activating the cytotoxicity of IELs against enterocytes.
As far as we know, it is not clear whether RCD1 and RCD2 are 2 different phases of the same condition or 2 independent conditions. Because a progression of RCD1 into RCD2 has been described in only 2 patients9 and EATL has been described in only 2 patients with RCD1,7 RCD1 and RCD2 may be 2 unrelated conditions.
Diagnosis
Clinical presentation
EATL manifests in adult patients with previously diagnosed CD, successfully treated until then with a strict GFD (secondary EATL), as an exacerbation of the classic symptoms of CD, such as abdominal pain, diarrhea, and unexplained weight loss.1 The concomitant presence of fever and night sweating, together with laboratory parameters indicative for hypoalbuminemia, anemia, and increased lactate dehydrogenase (LDH), should alert physicians to this complication. The median age at diagnosis of EATL is 60 years, with similar frequency between men and women. On the other hand, EATL may also arise in patients without a known history of CD and on a gluten-containing diet (primary EATL), and in these cases the diagnosis is more difficult and delayed because of the low specificity of symptoms and a very low index of clinical suspicion.16 In the subjects with EATL identified before CD has been diagnosed, the link between CD and EATL may be suggested by the detection of CD-specific antibodies (either antiendomysial or antitransglutaminase), although the latter often disappear once the refractory state is fully developed.7,15
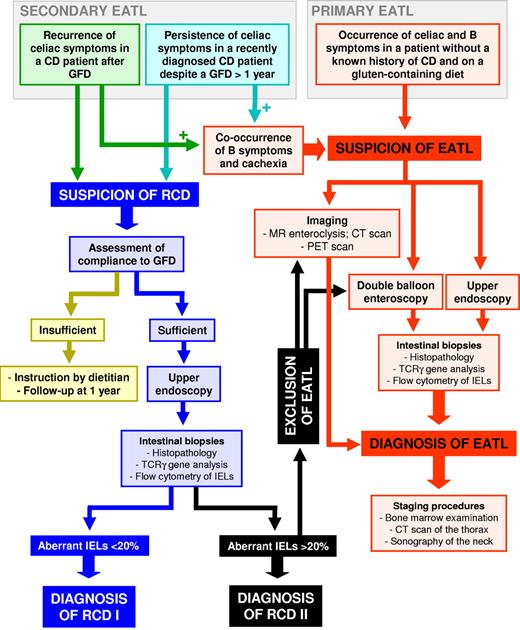
Because EATL may be complicated by gastrointestinal perforation, obstruction, or hemorrhage, many EATLs are diagnosed at laparotomy. At gross examination, EATL appears as a massive tumor infiltration, which may be transparietal, with ulcerations and induration of the intestinal wall. Up to 25% of cases have a multifocal presentation, and the proximal small bowel, particularly the jejunum, is a more common localization than the large bowel or rectum.25 There are reports of an association between EATL and peripheral eosinophilia,26 mesenteric lymph node cavitation,27 or splenic atrophy,28 the latter of which may increase susceptibility to severe infections or sepsis.29 Malnutrition is a common feature, especially when EATL has an insidious and chronic presentation or manifests after a long-standing RCD.16 Extraintestinal presentation of EATL is rare, and there is a lack of data on the precise characteristics of its cutaneous, neuromeningeal, or pulmonary manifestations. Systemic or B symptoms,30 such as fever of no evident cause, night sweats, and weight loss of more than 10% of body weight, should be taken as signs of clinical progression, although they occur in less than 30% of EATLs.16,31 A high level of clinical suspicion for an overt lymphoma should lead to an extensive workup, including abdominal imaging, endoscopy, and histologic examination of gut biopsies (Figure 2). Laparotomy with collection of full-thickness biopsy specimens may be necessary in some cases.
Proposed diagnostic algorithm in EATL. The recurrence of celiac symptoms in a patient with CD under GFD, or the persistence of celiac symptoms in a recently diagnosed CD patient despite a GFD followed for more than 12 months, should raise the suspicion of RCD. In that case, the compliance to GFD should be ascertained, and, if sufficient, patients should undergo upper endoscopy with collection of duodenal biopsies. A rate of more than 20% of aberrant IELs, assessed by flow cytometric analysis of freshly isolated cells, points toward a diagnosis of RCD2. In that case, EATL should be excluded through both imaging (either MR enteroclysis or CT scan together with PET scan) and double-balloon endoscopy with collection of small bowel biopsies. The occurrence of celiac and B symptoms (fever of no evident cause, night sweats, and weight loss of > 10% of body weight) in a patient without a known history of CD and on gluten-containing diet (primary EATL) or the co-occurrence of B symptoms and cachexia in the 2 aforementioned patient subsets suspected to be refractory (secondary EATL) should raise the suspicion of EATL. In that case, patients should undergo both upper endoscopy and double-balloon enteroscopy with collection of small bowel biopsies and imaging procedures (either MR enteroclysis or CT scan together with PET scan). In the case of EATL confirmation, a staging should be performed through bone marrow examination, CT scan of the thorax, and sonography of the neck.
Proposed diagnostic algorithm in EATL. The recurrence of celiac symptoms in a patient with CD under GFD, or the persistence of celiac symptoms in a recently diagnosed CD patient despite a GFD followed for more than 12 months, should raise the suspicion of RCD. In that case, the compliance to GFD should be ascertained, and, if sufficient, patients should undergo upper endoscopy with collection of duodenal biopsies. A rate of more than 20% of aberrant IELs, assessed by flow cytometric analysis of freshly isolated cells, points toward a diagnosis of RCD2. In that case, EATL should be excluded through both imaging (either MR enteroclysis or CT scan together with PET scan) and double-balloon endoscopy with collection of small bowel biopsies. The occurrence of celiac and B symptoms (fever of no evident cause, night sweats, and weight loss of > 10% of body weight) in a patient without a known history of CD and on gluten-containing diet (primary EATL) or the co-occurrence of B symptoms and cachexia in the 2 aforementioned patient subsets suspected to be refractory (secondary EATL) should raise the suspicion of EATL. In that case, patients should undergo both upper endoscopy and double-balloon enteroscopy with collection of small bowel biopsies and imaging procedures (either MR enteroclysis or CT scan together with PET scan). In the case of EATL confirmation, a staging should be performed through bone marrow examination, CT scan of the thorax, and sonography of the neck.
Staging
Staging procedures as recommended for lymphoma, such as bone marrow examination, CT scan of the thorax, and sonography of the neck, are required for therapeutic decision-making. However, classic lymphoma staging systems32,33 have failed in providing adequate prognostic treatment guidance in patients with EATL. The Lugano system30 is the staging method commonly used for intestinal lymphoma, although its prognostic value in EATL is uncertain. The Prognostic Index for Peripheral T-Cell Lymphoma34 was more predictive of survival than the International Prognostic Index,33 as 3 of the 5 components of the latter (ie, tumor stage, age, and number of extranodal sites) were irrelevant for EATL prognosis. Conversely, risk factors identified through the Prognostic Index for Peripheral T-Cell Lymphoma (ie, tumor size ≥ 5 cm, nonambulatory performance status, and raised C-reactive protein and LDH levels) were found to be significantly associated with a worse overall survival and failure-free survival in patients with EATL.34 A preliminary report35 identified 3 risk factors predictive for survival in a multivariate analysis (ie, raised LDH, presence of B-symptoms, and secondary EATL). This new prognostic model showed superior predictive capacity compared with the International Prognostic Index and made it possible to identify a high-risk group needing more aggressive therapies. It must be stressed, however, that all these prognostic studies were performed on patient series, which were either very heterogeneous or very small, besides being treated without intensified chemotherapy, and in a pre-autologous hematopoietic stem cell transplantation era. Thus, they can be of little help in selecting modern treatments.
Imaging and endoscopy
Although new imaging (MR enteroclysis, PET scan) and endoscopic techniques (wireless capsule endoscopy, double-balloon enteroscopy) able to visualize the entire small bowel are currently available,36 the exact diagnostic algorithm in EATL is still unclear because of the lack of studies aimed at comparing the accuracy of the different diagnostic modalities.37 Abdominal CT scan has a higher accuracy than small bowel enema in detecting EATL, which appears as a thickened small bowel loop with possible signs of mucosal ulcerations. Moreover, CT scan permits the visualization of extraintestinal findings, and it was proposed that the detection of bowel wall thickening, mesenteric lymph node cavitation, intussusception, and small-sized spleen (< 120 cm3) should raise suspicion for RCD2 or EATL.38 PET scan has a high sensitivity in detecting EATL (Figure 3),39 but active peristalsis and inflammation may affect its specificity.40 MR imaging is particularly useful in detecting EATL confined to the epithelial layer of the bowel wall or multifocal, and in assessing the response to treatment.41 The diagnostic accuracy of MR enteroclysis in detecting RCD2 or EATL was estimated to be almost 90%.42
Features of EATL at PET scan. Intensely increased 18F-FDG-PET uptake areas (arrows) are evident on axial, sagittal, and coronal images of the small bowel of a patient with EATL.
Features of EATL at PET scan. Intensely increased 18F-FDG-PET uptake areas (arrows) are evident on axial, sagittal, and coronal images of the small bowel of a patient with EATL.
Diagnostic accuracy can be further improved by wireless capsule endoscopy, which makes it possible to assess the extent of small bowel involvement and the presence of ulcerations, and is also a well-tolerated procedure, although contraindicated in case of suspected strictures.43 Double-balloon enteroscopy may allow a definitive diagnosis of EATL thanks to the collection of biopsy specimens.44 A recent study45 has shown that double-balloon enteroscopy is able to identify even diminutive small bowel lesions (ie, multiple polyps, fine granular mucosa, and swollen folds), thus suggesting a promising role for this technique in diagnosing EATL at an early stage.
Pathology
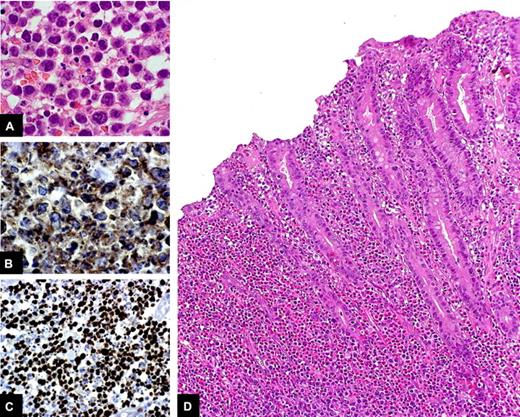
Histologic examination of small intestinal biopsies remains crucial in the diagnostic workup of EATL. Two different types of EATL are recognized on the basis of the specific immunophenotype: EATL type 1, which is characterized by CD56 negativity; and EATL type 2, which shows CD56 expression.18 EATL type 1, which is more frequent than EATL type 2 and is the only one associated with CD, is composed of CD3+,CD4−, CD8−,CD5−, CD7+,CD103+,CD56−,TCRβ+/− cells, which mostly express CD30. The cytotoxic phenotype of EATL type 1 cells, which are perforin+, granzyme B+, and TIA-1+, reflects the origin of the tumor from cytotoxic IELs.46,47 EATL type 1 mostly consists of medium-sized to large tumor cells with round or angulated vesicular nuclei, prominent nucleoli, and pale-staining cytoplasm and an increased mitotic index, often associated with a moderate to abundant infiltrate of eosinophils, histiocytes, and small lymphocytes (Figure 4A-C).25 The neighboring mucosa shows histologic features of active CD, such as crypt hyperplasia, increased IEL infiltration, and villous atrophy (Figure 4D). EATL type 2 is characterized by a monomorphic infiltrate of small- to medium-sized CD3+,CD4−, CD8+, CD56+, TCRβ+ cells. CD30 is often negative in EATL type 2, and the CD56 positivity suggests that a different mechanism underlies the lymphomagenic process of this tumor, which indeed is less commonly associated with CD.18
Histologic features of EATL. Medium-sized to large tumor cells with hyperchromatic nuclei, prominent nucleoli, and moderate to abundant, pale-staining cytoplasm, intermingled with eosinophils, are evident in the small bowel tissue specimen of a patient with EATL (A: H&E stain, original magnification ×100). The lesion is characterized by a considerable proportion of CD3-positive cells (B: CD3 immunostain, original magnification ×40) and a high proliferative rate (C: Ki-67 immunostain, original magnification ×40). At a lower magnification (D: H&E stain, original magnification ×20), it can be seen that the mucosa adjacent to the tumor shows histologic features of active CD, such as crypt hyperplasia, increased IEL infiltration, and villous atrophy.
Histologic features of EATL. Medium-sized to large tumor cells with hyperchromatic nuclei, prominent nucleoli, and moderate to abundant, pale-staining cytoplasm, intermingled with eosinophils, are evident in the small bowel tissue specimen of a patient with EATL (A: H&E stain, original magnification ×100). The lesion is characterized by a considerable proportion of CD3-positive cells (B: CD3 immunostain, original magnification ×40) and a high proliferative rate (C: Ki-67 immunostain, original magnification ×40). At a lower magnification (D: H&E stain, original magnification ×20), it can be seen that the mucosa adjacent to the tumor shows histologic features of active CD, such as crypt hyperplasia, increased IEL infiltration, and villous atrophy.
A PCR analysis indicative for a monoclonal rearrangement of the TCR-γ chain on intestinal tissue sections and an immunohistochemical or flow cytometric characterization of IELs indicative for an aberrant T-cell population have a high predictive value in identifying the progression of RCD into EATL48 and therefore can be of help in the diagnostic workup before an overt lymphoma has developed. Among the methods used to detect monoclonal rearrangement of TCR-γ genes, PCR has the advantage that it can be applied to formalin-fixed, paraffin-embedded specimens,49 unlike Southern blotting on extracted DNA, which requires fresh frozen tissue.50 TCR-γ clonal amplification has been identified in the duodenal biopsies of patients with RCD2 and UJI as in the subsequent tumor specimens.51,52 These findings further strengthen the concept that RCD2 and UJI can be regarded as “cryptic lymphomas” and should encourage performing an extensive diagnostic workup to exclude an overt lymphoma in both these premalignant conditions.
As far as the analysis of IEL phenotype is concerned, it has been shown that immunohistochemistry allows the discrimination between uncomplicated CD and RCD through the identification in the latter of aberrant IELs expressing a cytoplasmic CD3 chain but lacking CD3 and CD8 surface expression.53 However, flow cytometric analysis of IELs freshly isolated from intestinal biopsy specimens and permeabilized for CD3 cytoplasmic staining seems to be preferable to immunohistochemistry in the discrimination of RCD1 and RCD2 according to the cut-off value of 20% aberrant IELs (cytoplasmic CD3+, surface CD3−, CD7+, CD103+, CD8−, CD4−) proposed by Verbeek et al,48 although this technique is available only in tertiary referral centers. The main clinical, laboratory, and pathologic parameters, which guide us in the diagnostic management of patients with EATL, are summarized in Table 1.
Clinical, laboratory, and pathology findings in EATL
| Intestinal symptoms |
| Abdominal pain |
| Diarrhea |
| Systemic or B symptoms |
| Fever |
| Night sweating |
| Unexplained weight loss |
| Gastrointestinal complications |
| Perforation |
| Obstruction |
| Hemorrhage |
| Comorbidity |
| Splenic hypofunction/atrophy |
| Mesenteric lymph node cavitation |
| Severe malnutrition |
| Laboratory findings |
| Anemia |
| Peripheral eosinophilia |
| Low albumin |
| High lactate dehydrogenase |
| Pathology findings |
| CD3 positivity/CD4 negativity |
| High mitotic index |
| Nonmonomorphic infiltrate |
| CD56 negativity |
| CD30 positivity |
| High eosinophils, histiocytes, and small lymphocytes |
| Monoclonal rearrangement of the TCR-γ chain at PCR analysis |
| Intestinal symptoms |
| Abdominal pain |
| Diarrhea |
| Systemic or B symptoms |
| Fever |
| Night sweating |
| Unexplained weight loss |
| Gastrointestinal complications |
| Perforation |
| Obstruction |
| Hemorrhage |
| Comorbidity |
| Splenic hypofunction/atrophy |
| Mesenteric lymph node cavitation |
| Severe malnutrition |
| Laboratory findings |
| Anemia |
| Peripheral eosinophilia |
| Low albumin |
| High lactate dehydrogenase |
| Pathology findings |
| CD3 positivity/CD4 negativity |
| High mitotic index |
| Nonmonomorphic infiltrate |
| CD56 negativity |
| CD30 positivity |
| High eosinophils, histiocytes, and small lymphocytes |
| Monoclonal rearrangement of the TCR-γ chain at PCR analysis |
Genetics
Homozygosity for HLA-DQ2 (HLA-DB1*02) and allelic variants of the MYO9B gene region have been found to be associated with EATL.54,55 Moreover, data from comparative genomic hybridization studies on tumor DNA suggest that chromosomal gains of 1q and 5q and segmental amplification of 9q or deletion in 16q are associated with EATL.56,57 However, we do not routinely detect these findings in patients with EATL.
Treatment
A validated standard treatment of EATL in patients with CD is still lacking. The low incidence of this malignancy, the wide spectrum of its clinical presentation, its complex diagnosis, and the frequently poor performance status of the host because of malnutrition hamper the development of prospective controlled or randomized clinical trials.
Surgery
The therapeutic role of surgery lies in local debulking and in resection of tumor masses with a high risk of obstruction or hemorrhage or perforation, which might become higher during chemotherapy or radiotherapy. A complication often represents the revealing event of the underlying lymphoma, and the consequent emergency surgery also has an important diagnostic role.31 A possible drawback of intestinal surgery has been indicated in the delayed start of chemotherapy, especially when postoperative fistulas or infections or problems of wound healing occur. Data from reports on single cases or a few patients would suggest a better prognosis and a minor risk of perforation in patients who have undergone complete resection compared with those who have residual disease.31,58-61
Standard-dose chemotherapy
Most of the reports are retrospective, monocentric, and with treatment schedules that have changed over time. The estimated median survival is 7.5 months, whatever the stage of the lymphoma at onset.16,31,58,62-64 Standard-dose multidrug chemotherapy is the treatment most widely used in clinical practice for EATL. However, after the diagnosis has been ascertained, chemotherapy cannot be administered in more than 50% of cases because of low performance status, mainly due to preexisting unresponsive CD, lymphoma dissemination, and frequently advanced age; moreover, a further 50% of those patients who begin chemotherapy are not able to complete it because of complications or disease relapse or iatrogenic toxicity. No more than 35% to 40% of the patients who complete chemotherapy achieve complete remission of the lymphoma16,31,58,63 ; the overall response rate ranges from 40% to 60%, with a slight and hardly valuable advantage for patients with early-stage lymphoma compared with those with advanced stage disease. The median duration of complete remissions is nearly 6 months,16,64 without any statistically significant difference between clinical stages. The only exception is represented by the 28 cases with EATL of the German Group,63 which showed a median remission duration of 28 months (range, 17-39 months) and a median 2-year survival of 28% (range, 13%-43%) with a clear, though statistically not significant, difference between stages. The CHOP chemotherapy regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) is the one most widely used and generally responsible for an overall 5-year survival of 9% to 22%.16,31,62-64 Many other chemotherapy regimens have been used instead of CHOP (Table 2), such as BACOP (bleomycin, doxorubicin, cyclophosphamide, vincristine, and prednisone),62 ProMACE-MOPP (prednisone, doxorubicin, cyclophosphamide, etoposide, mechlorethamine, vincristine, and procarbazine),62 VAMP (vincristine, doxorubicin, high-dose methotrexate, and prednisolone),16 PEACE-BOM (prednisolone, etoposide, doxorubicin, cyclophosphamide-bleomycin, vincristine, and methotrexate),16 and CHOEP (CHOP plus etoposide).63 All these regimens share 2 to 4 cornerstone drugs present in the CHOP schedule, with proven activity in lymphoproliferative diseases, and their efficacy proves similarly disappointing, with heavier toxicity if dose intensity is increased (BACOP) or other drugs are included (ProMACE-MOPP, PEACE-BOM, and CHOEP). Very few long-term survivors have been reported: 5 patients from the series of Gale et al with survival from 49 to 219 months after CHOP,16 and 1 patient from Hönemann et al with persisting complete remission 34 months after the end of the intensive Hyper-CVAD regimen (cyclophosphamide, vincristine, dexamethasone, methotrexate, and cytarabine).61 These long-term survivors, who often, but not always, present early-stage disease at diagnosis, suggest that a study of the prognostic factors of EATL might be suitable if a sufficiently high number of patients were available.
Results of the treatment with standard-dose chemotherapy
| Study type . | Reference . | No. of patients . | No. operated . | No. chemotherapy-treated . | Type of chemotherapy . | Overall response (complete response), % . | Median survival, mo (range) . |
|---|---|---|---|---|---|---|---|
| Retrospective | 62 | 24 | 12 | 11 | CHOP, BACOP, ProMACE-MOPP | NG (NG) | 10 (0-196) |
| Retrospective | 16 | 31 | 25 | 24 | CHOP, VAMP, PEACE-BOM | 58 (32) | 7.5 (0-83) |
| Retrospective | 61 | 1 | 1 | 1 | Hyper-CVAD | 100 (100) | 34 |
| Retrospective | 31 | 10 | 7 | 10 | CHOP | NG (50) | 5 (1-12) |
| Retrospective | 64 | 54 | 49 | 31 | CHOP, CHOP-like | 32 (32) | 7 (0-140) |
| Prospective | 58 | 23 | 13 | 15 | CHOP | NG (35) | 7 (NG) |
| Prospective | 63 | 10 | 10 | 10 | CHOEP | 60 (30) | 7 (2-16) |
| Study type . | Reference . | No. of patients . | No. operated . | No. chemotherapy-treated . | Type of chemotherapy . | Overall response (complete response), % . | Median survival, mo (range) . |
|---|---|---|---|---|---|---|---|
| Retrospective | 62 | 24 | 12 | 11 | CHOP, BACOP, ProMACE-MOPP | NG (NG) | 10 (0-196) |
| Retrospective | 16 | 31 | 25 | 24 | CHOP, VAMP, PEACE-BOM | 58 (32) | 7.5 (0-83) |
| Retrospective | 61 | 1 | 1 | 1 | Hyper-CVAD | 100 (100) | 34 |
| Retrospective | 31 | 10 | 7 | 10 | CHOP | NG (50) | 5 (1-12) |
| Retrospective | 64 | 54 | 49 | 31 | CHOP, CHOP-like | 32 (32) | 7 (0-140) |
| Prospective | 58 | 23 | 13 | 15 | CHOP | NG (35) | 7 (NG) |
| Prospective | 63 | 10 | 10 | 10 | CHOEP | 60 (30) | 7 (2-16) |
BACOP indicates bleomycin, doxorubicin, cyclophosphamide, vincristine, prednisone; C, complete response; CHOP, cyclophosphamide, doxorubicin, vincristine, prenisone; CHOEP, same drugs as in CHOP plus etoposide; CT, chemotherapy; Hyper-CVAD: cyclophosphamide, vincristine, dexamethasone, methotrexate, cytarabine; NG, not given; O, overall response; PEACE-BOM, prednisolone, etoposide, doxorubicin, cyclophosphamide-bleomycin, vincristine, methotrexate; ProMACE-MOPP, prednisone, doxorubicin, cyclophosphamide, etoposide, mechlorethamine, vincristine, procarbazine; and VAMP, vincristine, doxorubicin, high-dose methotrexate, prednisolone.
In our experience, patients who fail their first-line CHOP have a very dismal fate with a second-line conventional chemotherapy. In the experience of Raderer et al,59 6 of 19 patients underwent new chemotherapy regimens because of progressive disease or relapse. The regimens used were ICE (ifosfamide, carboplatin, and etoposide) in 2 patients, FC (fludarabine and cyclophosphamide) in another 2 subjects, cladribine in one, and DHAP (dexamethasone, cytarabine, and cisplatin) in the last. Three subjects responded completely (2 to FC and one to ICE), and 2 of them were still alive at 7 and 64 months, respectively; 1 died of disease progression after 10 months. All 3 nonresponders died of perforation within 2 weeks from the start of the second-line therapy.
High-dose chemotherapy with ASCT
The poor results of conventional chemotherapy have stimulated investigation into the feasibility and efficacy of high-dose chemotherapy followed by autologous stem cell transplantation (ASCT). This therapeutic option cannot be proposed to the large majority of patients because of their poor general condition, unresponsiveness to debulking therapy, iatrogenic toxicity and/or complications, or too early relapse. The series with the relatively highest number of treated patients are those by Jantunen et al (5 patients; median survival, 2 months),65 Bishton and Haynes (6 patients; 4 alive and disease-free 2-4 years after treatment,2 relapsed early),66 Reimer et al (5 patients with EATL, without any specific information, of 83 cases with different T-cell histology, 55 of whom were transplanted),67 and Al-Toma et al (4 patients; 1 had ongoing complete remission 32 months after transplantation, 3 died from relapse within a few months after transplantation).68 Table 3 summarizes all the information available in the literature, including case reports. CHOP was generally used as the first-line chemotherapy and BEAM (carmustine, etoposide, cytarabine, and methotrexate) as the conditioning regimen. A novel first-line chemotherapy, consisting of a single course of CHOP plus 6 cycles of alternating IVE (ifosfamide, epirubicin, etoposide) and MTX (intermediate-dose methotrexate), followed by either BEAM or high-dose melphalan with total body irradiation as conditioning regimens, was planned by Sieniawski et al and administered in 26 patients and compared with another 35 who underwent standard chemotherapy or surgery alone.64 The eligibility criteria were de novo onset of the EATL, age of 18 years or older, and estimated ability to tolerate high-dose treatment. The number of patients excluded from the enrollment was not specified, and only 14 of the 26 included in the study actually underwent ASCT. The study was not randomized, but the improvement of 5-year overall survival and progression-free survival was impressive (60% vs 22% and 52% vs 22%, respectively). A single report69 of prolonged complete remission of a refractory EATL after reduced-intensity unrelated cord blood transplantation offers new outlooks on the efficacy of conditioning procedures with lower toxicity and a probable graft-versus-lymphoma activity. On the whole, these studies suggest that those patients who can tolerate more intensive treatments may benefit. We think that high-dose chemotherapy followed by ASCT represents a true landmark in the evolving treatment of EATL, but we recommend a very strict selection of the eligible patients because of their intrinsic frailty.
Results of the treatment with high-dose chemotherapy followed by stem cell transplantation
| Study type . | Reference . | No. transplanted . | No. evaluable . | Debulking chemotherapy . | Conditioning treatment . | Complete response . | Median survival, mo (range) . |
|---|---|---|---|---|---|---|---|
| Retrospective | 16 | 2 | 2 | PEACE-BOM | BEAM | 2 | 31 (2-64) |
| Retrospective | 69 | 1 | 1 | EPOCH-ICE | MPH-FARA | 1 | 11 |
| Retrospective | 72 | 2 | 2 | CHOP, MACOP-B | BEAM, BEAM-like | NG | NG |
| Retrospective | 70 | 1 | 1 | CHOP-ESHAP | MCVC | 0 | 8 |
| Retrospective | 66 | 6 | 6 | IVE-HDMTX | BEAM | 5 | 40 (3-52) |
| Retrospective | 71 | 1 | 1 | CHOP | BEAM | 1 | 18 |
| Retrospective | 68 | 4 | 4 | CHOP | BEAM or MPH-FARA | 1 | 8 (2-34) |
| Retrospective | 65 | 5 | 5 | CHOP | BEAM, BEAM-like | NG | 2 (0.5-14) |
| Retrospective | 74 | 1 | 1 | CHOP | BEAM | 1 | 24 |
| Retrospective | 73 | 2 | 0 | PACEB-IVAM-HAM | BEAM | 1 | NG |
| Prospective | 67 | 5 | 0 | CHOP + DACMEMP (or PAEM) | TBI + HDCTX | NG | NG |
| Prospective | 64 | 14 | 14 | CHOP-IVE/MTX | TBI + HDMPH or BEAM | 10 | 60% at 5 y |
| (1-140+) | |||||||
| Prospective | 75 (RCD) | 13 | 13 | MPH-FARA | 5 | 66% at 4 y | |
| (10-67) |
| Study type . | Reference . | No. transplanted . | No. evaluable . | Debulking chemotherapy . | Conditioning treatment . | Complete response . | Median survival, mo (range) . |
|---|---|---|---|---|---|---|---|
| Retrospective | 16 | 2 | 2 | PEACE-BOM | BEAM | 2 | 31 (2-64) |
| Retrospective | 69 | 1 | 1 | EPOCH-ICE | MPH-FARA | 1 | 11 |
| Retrospective | 72 | 2 | 2 | CHOP, MACOP-B | BEAM, BEAM-like | NG | NG |
| Retrospective | 70 | 1 | 1 | CHOP-ESHAP | MCVC | 0 | 8 |
| Retrospective | 66 | 6 | 6 | IVE-HDMTX | BEAM | 5 | 40 (3-52) |
| Retrospective | 71 | 1 | 1 | CHOP | BEAM | 1 | 18 |
| Retrospective | 68 | 4 | 4 | CHOP | BEAM or MPH-FARA | 1 | 8 (2-34) |
| Retrospective | 65 | 5 | 5 | CHOP | BEAM, BEAM-like | NG | 2 (0.5-14) |
| Retrospective | 74 | 1 | 1 | CHOP | BEAM | 1 | 24 |
| Retrospective | 73 | 2 | 0 | PACEB-IVAM-HAM | BEAM | 1 | NG |
| Prospective | 67 | 5 | 0 | CHOP + DACMEMP (or PAEM) | TBI + HDCTX | NG | NG |
| Prospective | 64 | 14 | 14 | CHOP-IVE/MTX | TBI + HDMPH or BEAM | 10 | 60% at 5 y |
| (1-140+) | |||||||
| Prospective | 75 (RCD) | 13 | 13 | MPH-FARA | 5 | 66% at 4 y | |
| (10-67) |
EPOCH-ICE indicates etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, ifosfamide, carboplatin, and etoposide; MPH-FARA, melphalan and fludarabine; MACOP-B, methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin; NG, not given; ESHAP, etoposide, methylprednisolone, cytarabine, and cisplatin; MCVC, ranimustine, carboplatin, etoposide, and cyclophosphamide; IVE-HDMTX, ifosfamide, etoposide, epirubicin, and high-dose methotrexate; PACEB, doxorubicin, cyclophosphamide, etoposide, bleomycin, vincristine, and prednisone; IVAM, ifosfamide, etoposide, cytosine arabinoside, and methotrexate; HAM, cytarabine and mitoxantrone; DACMEMP, dexamethasone, cytarabine, carmustine, etoposide, methylprednisolone, and cisplatin; PAEM, cisplatin, cytarabine, etoposide, and methylprednisolone; TBI, total body irradiation; HDCTX, high-dose cyclophosphamide; and HDMPH, high-dose melphalan.
Radiotherapy
The administration of radiotherapy is seldom adopted in the treatment of patients with EATL and is reported as an occasional and ancillary measure related to chemotherapy. In the series by Novakovic et al, 6 of the 11 patients who completed the chemotherapy courses (4 with complete remission, 2 with partial response) were then treated with radiotherapy, and all achieved complete remission.31 In the study reported by Daum et al, none of the patients with EATL was able to receive radiotherapy after chemotherapy, as scheduled, because of progressive disease or complications.58
Novel treatments
An interesting new treatment is alemtuzumab, a monoclonal antibody that targets CD52 on normal and pathologic T lymphocytes. Gallamini et al treated 24 patients with different histologic types of T-cell lymphoma with a combination of CHOP and alemtuzumab.76 The only patient presenting an EATL responded completely, but no information was given about the duration of response. Soldini et al successfully treated a 73-year-old woman with a typical history of CD and subsequent EATL, administering alemtuzumab in combination with gemcitabine; the patient relapsed one year after and achieved a second durable complete remission with the same combined therapy.77 On the other hand, a gastric EATL in a 72-year-old man, treated by Kircher et al with CHOP plus alemtuzumab, showed an initial promising clinical and radiologic response but then rapidly progressed with a lethal outcome.78 Alemtuzumab was given at 30 mg 3 times a week for 4 consecutive weeks combined with antiviral therapy. Interestingly, alemtuzumab can prevent the risk of developing an EATL in subjects with RCD, as documented by the complete clinical and pathologic recovery of a woman with a 2-year history of RCD.79 Another single experience, however, did not confirm such efficacy.80
The clinical activity of cladribine, a synthetic purine nucleoside with cytotoxic effects, holds promise in the field of RCD and EATL. Cladribine has been used more in RCD2. In this setting of patients, the pilot study by Al-Toma et al recorded 6 of 17 clinical responses, with histologic improvement in 10 of 17 after 1 course of therapy (0.1 mg/kg with a 2-hour infusion daily for 5 days).81 These results were confirmed by Tack et al in 32 patients with RCD2 because 18 of 37 responders had a clearly higher 5-year survival and a decrease transition into EATL compared with nonresponders.82 Patients unresponsive to cladribine can be rescued with high-dose chemotherapy and stem cell transplantation.75 No information is available on the use of cladribine in overt EATL apart from the report by Raderer et al regarding a patient with relapsed and unresponsive EATL.59
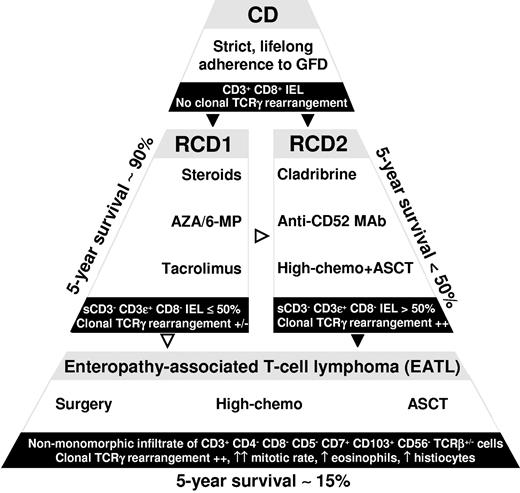
A complete response, stable 8 months after treatment, was achieved in a patient with EATL also by romidepsin as a single agent, a histone deacetylase inhibitor active in T-cell lymphoma, administered in a phase 2 study83 on different types of T-cell lymphomas. Finally, laboratory studies are ongoing on the activity in vitro and in mice of a fully humanized IL-15 specific antibody (AMG714)24 able to block the signaling pathway by which IL-15 transmits antiapoptotic signals to IELs. It is probable that an antibody like this, which specifically acts on one crucial step of the lymphomagenesis, may be the conclusive weapon against EATL if used in RCD with the aim of preventing the malignant transformation of IELs. Figure 5 summarizes the main therapeutic options we adopt in patients with EATL or RCD.
Main therapeutic options (white areas), 5-year survival, and IEL or infiltrating lymphoma cell immunophenotype (black areas), along the lymphomagenic process leading from uncomplicated CD to EATL through RCD. Unlike RCD1 (RCD1), which can benefit from steroids, such as prednisone or budesonide, combined or not with immunomodulators, such as azathioprine (AZA), 6-mercaptopurine (6-MP), or tacrolimus, RCD2 (RCD2) is less likely to respond to the aforementioned drugs. Therapeutic options in RCD2 may include cladribrine, alemtuzumab (anti-CD52 antibody), or high-dose chemotherapy (high-chemo) followed by ASCT. Standard-chemo indicates standard-dose chemotherapy. Black arrowheads represent probable progression; and white arrowheads, uncertain progression.
Main therapeutic options (white areas), 5-year survival, and IEL or infiltrating lymphoma cell immunophenotype (black areas), along the lymphomagenic process leading from uncomplicated CD to EATL through RCD. Unlike RCD1 (RCD1), which can benefit from steroids, such as prednisone or budesonide, combined or not with immunomodulators, such as azathioprine (AZA), 6-mercaptopurine (6-MP), or tacrolimus, RCD2 (RCD2) is less likely to respond to the aforementioned drugs. Therapeutic options in RCD2 may include cladribrine, alemtuzumab (anti-CD52 antibody), or high-dose chemotherapy (high-chemo) followed by ASCT. Standard-chemo indicates standard-dose chemotherapy. Black arrowheads represent probable progression; and white arrowheads, uncertain progression.
Prophylaxis: the role of a gluten-free diet
Adherence to a strict GFD is of paramount importance in reducing the risk of developing CD complications, and its protective effect was shown more than 20 years ago; in the past few years, many papers have addressed this issue, confirming its importance.84-91 However, on the basis of a reduced malignancy risk in asymptomatic celiac patients diagnosed because of familiarity compared with celiac patients diagnosed because of symptoms, it has recently been suggested that there is no evidence that a GFD further reduces the risk of malignant complications in asymptomatic celiac patients.92 Until these results are confirmed, we strongly recommend that all celiac patients must maintain a strict life-long GFD.
In conclusion, EATL is a rare disease with a very poor outcome. Unexplained weight loss, abdominal pain, diarrhea, loss of albumin and blood, increased LDH, fever, and night sweating should alert physicians to this complication in patients with a history of CD or RCD. However, EATL may also be diagnosed in patients without a known history of CD. The suspicion of EATL should lead to an extensive diagnostic workup in which MR enteroclysis, combined with PET scan and histologic identification of lesions, represents the best options. The best current treatment choice is high-dose chemotherapy, preceded by surgical resection and followed by ASCT, although this procedure can only be applied to a strictly selected number of patients able to tolerate it. Further studies are needed to verify whether innovative therapies might be of help in treating or preventing EATL. Strict adherence to a GFD remains the best option to prevent EATL in patients with CD.
Acknowledgments
The authors thank Dr Vincenzo Villanacci for providing the histologic pictures shown in Figure 4, and the Associazione Italiana Celiachia for supporting them in the study of complicated celiac disease.
Authorship
Contribution: A.D.S., F.B., P.G.G., and G.R.C. synthesized ideas and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gino R. Corazza, Clinica Medica I, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo, Università di Pavia, Piazzale Golgi 5, 27100 Pavia, Italy; e-mail: gr.corazza@smatteo.pv.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal