Abstract
Immature platelets (IPFs), which are hemostatically more active than mature platelets, have been found elevated in essential thrombocythemia and polycythemia vera, 2 myeloproliferative neoplasms (MPN) characterized by an increased risk of thrombosis. It is not known whether the IPF levels are influenced by pathogenetic factors, including JAK2V617F mutational status, or by treatment regimen. To address this point, in 46 essential thrombocythemia and 38 polycythemia vera consecutive patients, we measured IPF and correlated the results to JAK2V617F mutation and myelosuppressive treatment with hydroxyurea. This analysis provides 2 new elements regarding IPF and MPN. The first finding is that the JAK2V617F mutation is linked to the quantity of IPF in patients with MPN, which might contribute to the prothrombotic phenotype in these patients. The second finding is that IPF is susceptible to myelosuppressive treatment, which may additionally explain the favorable effect of hydroxyurea therapy on MPN outcome as well as the associated thrombotic risk.
Introduction
Circulating platelets are heterogeneous in size and structure. A small percentage of circulating platelets (ie, 2%) are the so-called reticulated or immature platelets (IPFs), recently released from the bone marrow. In vitro studies show that newly formed murine platelets have increased hemostatic activity compared with mature platelets, as demonstrated by the increased response to thrombin and higher expression of surface P-selectin.1
Elevated numbers of IPF population have been described in 2 myeloproliferative neoplasms (MPNs) characterized by an increased thrombotic risk (ie, essential thrombocythemia [ET] and polycythemia vera [PV]), as well as in other thrombotic disorders.2-4 It is not known whether this increase is influenced by pathogenetic factors, including JAK2V617F mutational status, or by treatment regimen in ET and PV patients. Therefore, in this study, we enrolled ET and PV patients to characterize immature platelet parameters according to JAK2V617F mutation and treatment.
Methods
IPFs were measured in whole blood by the fully automated hematology analyzer XE-2100 (Sysmex) and are described as total IPF count, percentage of IPF and H-IPF, the latter representing the percentage of platelets within the IPF area with a major amount of m-RNA and thus with the highest fluorescent intensity.5,6 The collection of venous blood samples and JAK2V617F mutation analysis were performed as previously described.7 All procedures were approved by the Institutional Review Board of the Ospedali Riuniti, Bergamo, Italy. All statistical analyses were done with SPSS Version 15 (SPSS). The results are presented as mean ± SD. The intergroup data comparisons were performed using the Student t test or the nonparametric Mann-Whitney-Wilcoxon U test, according to the distribution of the test variables. All reported P values are 2-sided with a type I error rate of 5%.
Results and discussion
This study was conducted in 42 healthy subjects and in a consecutive series of 46 ET and 38 PV patients. Twenty-three ET and 18 PV received cytoreductive therapy with hydroxyurea (HU), 35 (16 ET and 19 PV) were on aspirin alone, and 8 (7 ET and 1 PV) were not receiving any cytoreductive or aspirin treatment. Twenty-three ET patients (50%) and 34 PV patients (89%) have the JAK2V617F mutation. The mutation in all ET patients was heterozygous, whereas among PV patients, 27 were heterozygous and 7 were homozygous for the JAK2V617F mutation. Two PV patients, negative for JAK2V617F, were positive for the JAK2 exon12 mutation.
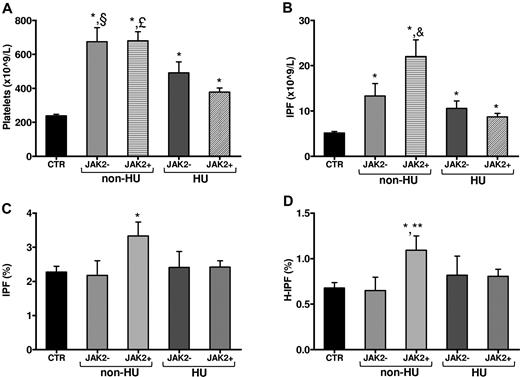
Both ET and PV patients had significantly higher platelet and IPF counts compared with controls (P < .01). In addition, PV patients had significantly (P < .01) higher hematocrit, white blood cell count, percentage of IPF, and percentage of H-IPF compared with both controls and ET patients. Compared with control subjects, JAK2V617F-positive patients showed significantly (P < .05) higher platelet (522 ± 260 vs 238 ± 58 × 109/L) and IPF count (15 ± 14 vs 5.0 ± 2.0 × 109/L), percentage of IPF (2.8% ± 1.6% vs 2.2% ± 1%), and percentage of H-IPF (0.9% ± 0.6% vs 0.6% ± 0.3%), whereas JAK2V617F-negative patients had significantly higher platelet (594 ± 283 × 109/L) and IPF counts (12 ± 8 × 109/L). Figure 1 shows the data after grouping the patients according to both JAK2V617F mutation and HU treatment. Non-HU JAK2V617F-positive patients showed significantly (P < .05) higher platelet count (Figure 1A), IPF count (Figure 1B), percentage of IPF (Figure 1C), and percentage of H-IPF (Figure 1D) compared with control subjects. In addition, non-HU JAK2V617F-positive patients showed significantly higher IPF count also compared with non-HU JAK2V617F-negative patients and HU-treated JAK2V617F-positive and -negative patients (Figure 1B). Within non–HU-treated subjects, JAK2V617F-positive patients showed a significantly higher percentage of H-IPF compared with JAK2V617F-negative patients (Figure 1D). Finally, within patients negative for JAK2V617F mutation, no significant differences in IPF parameters were observed between HU and non–HU-treated patients. Differently, within patients positive for JAK2V617F mutation, HU-treated patients showed significantly lower (P < .05) IPF parameters compared with non–HU-treated patients (IPF count: 8.7 ± 4 vs 22 ± 18 × 109/L; percentage of IPF: 2.4% ± 1.0% vs 3.3% ± 2.1%).
IPF parameters according to JAK2V617F mutation and therapy. Panels are showing: (A) platelet count, (B) IPF count, (C) percentage of IPF, and (D) percentage of H-IPF. Data are mean ± SD. CTR indicates controls; JAK2+, JAK2V617F-positive patients; JAK2−, JAK2V617F-negative patients. *P < .05 vs CTR. §P < .05 vs HU JAK2+. £P < .05 vs HU JAK2+, HU JAK2−. &P < .05 vs non-HU JAK2−, HU JAK2−, HU JAK2+. **P < .05 vs non-HU JAK2−.
IPF parameters according to JAK2V617F mutation and therapy. Panels are showing: (A) platelet count, (B) IPF count, (C) percentage of IPF, and (D) percentage of H-IPF. Data are mean ± SD. CTR indicates controls; JAK2+, JAK2V617F-positive patients; JAK2−, JAK2V617F-negative patients. *P < .05 vs CTR. §P < .05 vs HU JAK2+. £P < .05 vs HU JAK2+, HU JAK2−. &P < .05 vs non-HU JAK2−, HU JAK2−, HU JAK2+. **P < .05 vs non-HU JAK2−.
Multivariate regression analysis adjusted for age, sex, JAK2V617F mutational status, and HU therapy confirmed that JAK2V617F mutation is significantly associated (P < .01) with higher percentage of IPF (β = 0.42) and H-IPF (β = 0.38), and IPF count (β = 0.7); whereas HU treatment is significantly associated (P < .01) with lower percentage of IPF (β = −0.43) and H-IPF (β = −0.32), and IPF counts (β = −0.45).
The standard flow cytometric measurement of IPF is considered imprecise, time-consuming, and expensive; and most importantly, no interlaboratory consensus of the normal range has been obtained as yet.5,8 Therefore, we have evaluated the percentage of IPF in controls and patients by the recently established fully automated and rapid Sysmex XE-2100 method. The new XE-2100 expands the quantitative analytic range to reticulocytes, immature granulocytes, and optical fluorescent platelet count. The reference range in our study for the percentage of IPF in healthy subjects using this method was 0.7% to 6% with a mean value of 2.2%, which is in agreement with other studies.5,9
The measurement of IPF has been considered useful in assessing the treatment response and thrombotic risk in patients with thrombocytosis, both primary and secondary.10 Increased IPF has also been associated with increased thrombotic risk (ie, in acute coronary syndromes and in cardioembolic stroke).4,11 In addition, increased percentage of IPF and H-IPF has been associated with increased platelet aggregation in high-risk patients with coronary artery disease.6 Similarly, renal transplant recipients, who are at increased risk for cardiovascular mortality, showed higher percentage of IPF and H-IPF associated with increased platelet aggregation.12 Because of an increased platelet turnover, MPN patients have a higher percentage of IPF compared with healthy controls.3 In our study, we found that the absolute counts of IPF were significantly (P < .01) increased in both ET and PV patients compared with controls, whereas the percentage of both IPF and H-IPF was significantly increased in PV patients only. The analysis according to JAK2V617F mutational status and HU treatment showed that non–HU-treated MPN mutation-positive patients had significantly higher percentage of IPF and H-IPF compared with control subjects. These findings suggest that JAK2V617F mutation might be an important factor related to the increased percentage of IPF and H-IPF in MPN patients. The adjusted multivariate analysis demonstrating that JAK2V617F mutation is associated with increased IPF count and percentage of IPF and H-IPF confirmed our results. Interestingly, our study showed that, within patients positive for the JAK2V617F mutation, HU-treated patients have significantly lower IPF parameters compared with non–HU-treated patients, which was not observed within JAK2V617F-negative patients. This finding is in line with a recently published study showing in MPN subjects positive for JAK2V617F mutation an increased chemosensitivity to HU.13
In conclusion, this study provides 2 new elements regarding the association between IPF and MPN. The first finding is that the JAK2V617F mutation is linked to the quantity of IPF in patients with MPN, which might contribute to the prothrombotic phenotype in these patients. The second finding is that IPF is susceptible to HU treatment, which may additionally explain the favorable effect of this therapy on MPN as well as the associated thrombotic risk. New prospective studies are warranted to evaluate the usefulness of IPF as a cellular marker to predict thrombosis in MPN patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Institutes of Health (Myeloproliferative Disorders–Research Consortium grant), the Annadal Foundation (Maastricht, The Netherlands), and the Associazione Italiana per la Ricerca sul Cancro (Milan, Italy).
Authorship
Contribution: M.P.-N. designed and performed research, collected the clinical data, analyzed and interpreted data, and wrote the paper; M.M. designed research, analyzed and interpreted data, and wrote the paper; A.F. designed research, interpreted data, and contributed to writing the paper; H.t.C. contributed to writing the paper; S.B. performed research; L.R. and A.L. performed research and recruited healthy control subjects; A.R and G.F. contributed to patient recruitment; and C.O. contributed to critical review and discussion of the results.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anna Falanga, Division of Immunohematology and Transfusion Medicine, Ospedali Riuniti di Bergamo, Largo Barozzi, 1, 24128 Bergamo, Italy; e-mail: annafalanga@yahoo.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal