In this issue of Blood, Hirsch and colleagues demonstrate that granulocyte- colony stimulating factor (G-CSF) mobilization does not induce chromosomal aneuploidy or changes in allelic replication timing in healthy donors.1 This important negative study provides reassurance to the hematopoietic stem cell transplant (HSCT) community.
G-CSF is the principal regulator of granulopoiesis, and this cytokine is frequently administered to patients with severe neutropenia. An increased rate of clonal evolution to monosomy 7 myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) was observed in patients with severe aplastic anemia (SAA)2 and severe congenital neutropenia3 treated with G-CSF. However, this finding has not been consistent across all studies.4 G-CSF has also been associated with an increased risk of secondary MDS and AML in breast cancer patients treated with chemotherapy.5 These observations have raised the concern that G-CSF administration promotes clonal aneuploidy, MDS, and high-risk AML.
G-CSF is routinely administered to healthy donors to mobilize peripheral blood stem cells (PBSCs) for allogeneic HSCT. Prior studies have reported that G-CSF induces chromosomal aneuploidy and epigenetic changes in the peripheral blood of healthy HSCT donors. Nagler et al observed that G-CSF mobilization induces altered replication timing, or replication asynchrony, of the TP53, AML1, and CEN17 loci.6 Replication asynchrony of the TP53 and AML1 genes has been detected in the peripheral blood of patients with hematologic malignancies, and CEN17 is involved with segregation of chromosome 17. Nagler and colleagues also reported chromosome 17 aneuploidy in the peripheral blood of G-CSF mobilized healthy donors.6 Similarly, Marmier-Savet et al observed aneuploidy of chromosomes 8 and 17 in healthy donors after G-CSF mobilization.7 Neither study examined monosomy 7 aneuploidy, and these genomic and epigenetic changes were observed in cultured lymphocytes stimulated with phytohemaglutinin (PHA), rather than in CD34+ progenitors and stem cells. Nevertheless, these potentially worrisome findings have prompted the World Marrow Donor Association to recommend counseling prospective HSCT donors on the uncertainty as to whether G-CSF increases an individual's risk of malignancy.8 Thus, these findings may potentially reduce the number of donors for allogeneic HSCT.
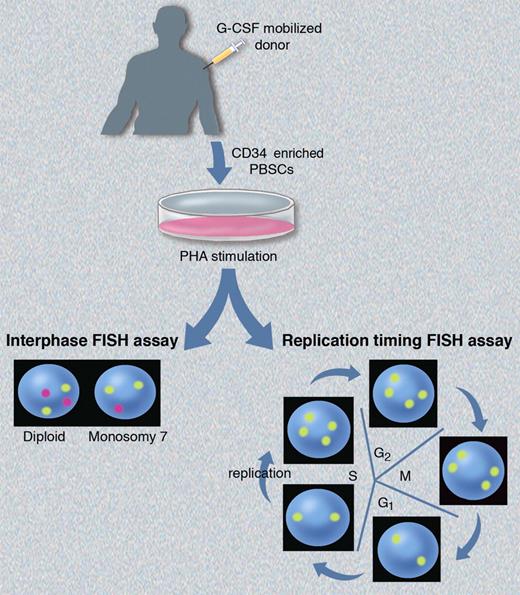
Here, Hirsch et al report on the first prospective, controlled study of chromosomal aneuploidy and replication asynchrony in G-CSF mobilized HSCT donors.1 They used FISH assays to assess aneuploidy and perturbations in replication timing, and their studies were performed using the same experimental approach as Nagler et al6 (see figure). CD34+ enriched cells collected from G-CSF–mobilized healthy donors were cultured with PHA to stimulate peripheral blood lymphocytes, or studied without prior in vitro culture. They performed interphase FISH using 3 probes specific for chromosome 7, and observed no increase in monosomy 7 aneuploidy in healthy donor PBSCs immediately after mobilization, or at time points up to one year after G-CSF administration compared with their baselines, or to untreated controls. They also found no increased aneuploidy of CEN17 as reported previously,6,7 or in the AML-associated genes RUNX1T1, TP53, and RUNX1. Aneuploidy levels varied among individual FISH probes, likely because of artifacts such as incomplete hybridization with large probes that bind to polymorphic regions. These artifacts may account for the contrasting aneuploidy levels reported in prior studies.6,7
Schematic of the FISH assays used by Hirsch et al1 to assess chromosomal aneuploidy and allelic replication timing. Interphase FISH (bottom left) demonstrates the expected patterns for diploid and monosomy 7 cells using probes for chromosomes 8 (yellow) and 7 (magenta). The expected hybridization pattern for replication synchronous alleles using a single yellow probe will differ depending on where the cells are in cycle (bottom right). Postmitotic cells in G1 and early S phase (before replication) will exhibit a “singlet-singlet” signal corresponding to each allele, while cells that have undergone replication later in S phase will exhibit a “doublet-doublet” pattern corresponding to the 2 replicated alleles. Professional illustration by Marie Dauenheimer.
Schematic of the FISH assays used by Hirsch et al1 to assess chromosomal aneuploidy and allelic replication timing. Interphase FISH (bottom left) demonstrates the expected patterns for diploid and monosomy 7 cells using probes for chromosomes 8 (yellow) and 7 (magenta). The expected hybridization pattern for replication synchronous alleles using a single yellow probe will differ depending on where the cells are in cycle (bottom right). Postmitotic cells in G1 and early S phase (before replication) will exhibit a “singlet-singlet” signal corresponding to each allele, while cells that have undergone replication later in S phase will exhibit a “doublet-doublet” pattern corresponding to the 2 replicated alleles. Professional illustration by Marie Dauenheimer.
Hirsch and colleagues also performed replication timing FISH experiments.1 DNA replication occurs during S phase of the cell cycle according to a tightly regulated process that is temporally conserved between successive cell divisions with most allelic pairs replicating in synchrony. Alleles exhibiting replication asynchrony can be detected by stimulating the cells into cycle, and then performing FISH for loci of interest. Alleles exhibiting a delay in replication timing will deviate from the expected pattern (depicted in figure) with a “singlet-doublet” FISH signal in late S phase cells. Hirsch et al found no changes in replication timing of TP53 and 2 other alleles after G-CSF mobilization in HSCT donors followed out to 1 year.1
Hirsch and colleagues did not examine the effects of G-CSF mobilization on monosomy 7 aneuploidy in the bone marrow where clonal evolution to MDS originates. However, monosomy 7 MDS generally progresses to AML at a rapid pace, so it is unlikely that aneuploid clones in mobilized PBSCs would escape detection by FISH 12 months after initiation. Moreover, recent long-term epidemiologic studies in more than 6000 individuals have not found a higher than expected incidence of monosomy 7 MDS or AML in unrelated HSCT donors.9,10
From a clinical perspective, this study by Hirsch et al is important because these findings do not support previous observations suggesting that G-CSF mobilization causes genotoxic changes, including epigenetic changes seen in patients with hematologic malignancies. These results, taken with recent epidemiologic data,9,10 should assuage the concerns of potential HSCT donors, which may increase the pool of allografts in donor registries. These are welcome findings, given that HSCT remains the only curative therapy for many hematologic malignancies.
The finding that G-CSF promotes monosomy 7 aneuploidy in patients with marrow failure but not in healthy donors is intriguing. The duration of G-CSF exposure may not be the sole explanation, as this differential effect is also observed in vitro when bone marrows from healthy donors and those from patients with occult monosomy 7 clones are cultured with pharmacologic concentrations of G-CSF.11 Biologic phenomena such as perturbations in G-CSF receptor signaling11 may favor the expansion of monosomy 7 clones in some patients with marrow failure exposed to G-CSF. Further investigations along these lines may yield insights into mechanisms of clonal evolution and novel therapies for patients with monosomy 7 MDS.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal