The immunologic synapse is formed between a dendritic cell (DC) and a T cell and is critical for correct T-cell activation. In this issue of Blood, Bouma and colleagues define that the Wiskott-Aldrich syndrome protein (WASp) regulates assembly and function of the immunologic synapse in DCs.1
What makes highly motile T cells stop when they encounter an antigen-presenting cell in lymph nodes? The answer is the immunologic synapse formed at the interface between an antigen-presenting DC and an antigen-specific T cell.2 The immunologic synapse has been extensively characterized in T cells and is composed of a region of spatially and temporally organized adhesion proteins and receptors in supramolecular activation clusters (SMACs; see figure). A full understanding of the immunologic synapse requires analysis of both the T-cell side and the DC side of the synapse. However, the contribution of DCs to immunologic synapse assembly and function is largely unknown.
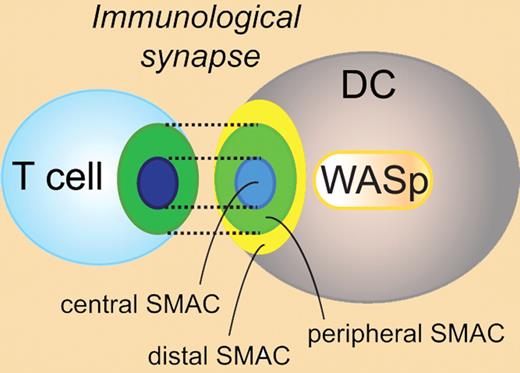
Schematic representation of the immunologic synapse between a T cell and a dendritic cell. WASp regulates the assembly and function of the immunologic synapse in DCs. DC indicates dendritic cell; SMAC, supramolecular activation cluster; and WASp, Wiskott-Aldrich syndrome protein.
Schematic representation of the immunologic synapse between a T cell and a dendritic cell. WASp regulates the assembly and function of the immunologic synapse in DCs. DC indicates dendritic cell; SMAC, supramolecular activation cluster; and WASp, Wiskott-Aldrich syndrome protein.
WASp has emerged as a critical regulator of the actin cytoskeleton in hematopoietic cells and regulates immunologic synapse stability in T cells.3 The importance of WASp activity for correct function of hematopoietic cells is revealed in patients with Wiskott-Aldrich syndrome who lack expression of WASp and suffer from severe immunodeficiency.4 Bouma et al investigated the role of WASp and the cell cytoskeleton at the DC side of the synapse. They show that disruption of the cell cytoskeleton and cellular signaling in WASp−/− DCs is enough to destroy the immunologic synapse at both the DC side and T-cell side of the synapse.1 Their data provide important insight into the active role of DCs for immunologic synapse assembly and function.
WASp−/− DCs have decreased migration and adhesion responses and reduced capacity to activate naive T cells.4 In the current investigation, Bouma et al show that WASp−/− DCs form less stable interactions with antigen-specific wild-type CD4+ T cells in lymph nodes. To exclude the migratory defect of WASp−/− DCs, the authors analyzed the DC-T synapse in vitro using time-lapse microscopy. They show that WASp−/− DCs form less stable interactions with antigen-specific wild-type CD4+ T cells also in vitro, resulting in decreased T-cell activation and proliferation. Polarization of the microtubuli organizing center toward the immunologic synapse is thought to be required for release of certain cytokines in the synapse interface.2 One such cytokine is IL-12, and a recent report showed that polarized secretion of IL-12 by DCs to the synapse is regulated by the WASp interacting protein Cdc42 and required for normal T-cell activation.5 WASp−/− DCs had normal polarization of the microtubule organizing center to the synapse, but reduced production of IL-12. It is tempting to speculate that WASp−/− DCs may fail to correctly activate T cells due to failure of targeting IL-12 secretion to the immunologic synapse.
Bouma et al next investigated the molecular structure of the synapse formed between WASp−/− DCs and wild-type T cells. Antigen-specific CD4+ T cells in contact with wild-type DCs showed the classic bull's-eye appearance of the synapse with large adhesion molecules such as CD45 and LFA-1 more distal and TCR in the central SMAC (see figure).2 In contrast, wild-type CD4+ T cells in contact with WASp−/− DCs failed to assemble the bull's-eye synapse and had decreased localized tyrosine phosporylation, a sign of diminished signaling at the synapse. To investigate the molecular structure at the DC side of the synapse, polarization of the LFA-1 adhesion partner I-CAM to the synapse was examined. Wild-type DCs in contact with antigen-specific CD4+ T cells had organized localization of I-CAM to the peripheral SMAC. Strikingly, WASp−/− DCs showed only diffuse I-CAM localization at the synapse interface.
The activity of WASp is regulated by its structural conformation and phosphorylation of a critical tyrosine residue (Y293).4 To determine how WASp regulates the DC immunologic synapse, Bouma et al investigate a WASp mutant strain that has abrogated phosphorylation of tyrosine-293 (Y293F). Similar to WASp−/− DCs, WASp-Y293F DCs showed diffuse polarization of I-CAM to the synapse interface, providing evidence that tyrosine phosphorylation of WASp is required for normal immunologic synapse assembly in DCs.
Finally, Bouma and colleagues translate their findings using murine WASp−/− DCs to DCs from a patient with Wiskott-Aldrich syndrome. The authors show that human WASp−/− DCs have reduced capacity to prime T cells as measured by IFNγ secretion.
Activation of antigen-specific T cells during an immune response depends on orchestral cycles of T-cell migration and stable immunologic synapse assembly with antigen-presenting DCs.2 On each T-cell migration phase, WASp regulates reassembly of synapse symmetry and stability in T cells.3 The present investigation by Bouma et al provides evidence that WASp serves a similar function in DCs. Future studies will define whether WASp regulates reassembly of the immunologic synapse in DCs after rounds of migration to find antigen-specific T cells. Bouma and colleagues provide new and important information about the myeloid deficiency in Wiskott-Aldrich syndrome. The reduced capacity of WASp−/− DCs to form a functional immunologic synapse with T cells has implications for the treatment of Wiskott-Aldrich syndrome patients and raises concerns for those patient who have limited myeloid reconstitution after bone marrow transplantation and gene therapy.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal