Abstract
Multiple distinct memory B-cell subsets have been identified in humans, but it remains unclear how their phenotypic diversity corresponds to the type of responses from which they originate. Especially, the contribution of germinal center-independent responses in humans remains controversial. We defined 6 memory B-cell subsets based on their antigen-experienced phenotype and differential expression of CD27 and IgH isotypes. Molecular characterization of their replication history, Ig somatic hypermutation, and class-switch profiles demonstrated their origin from 3 different pathways. CD27−IgG+ and CD27+IgM+ B cells are derived from primary germinal center reactions, and CD27+IgA+ and CD27+IgG+ B cells are from consecutive germinal center responses (pathway 1). In contrast, natural effector and CD27−IgA+ memory B cells have limited proliferation and are also present in CD40L-deficient patients, reflecting a germinal center-independent origin. Natural effector cells at least in part originate from systemic responses in the splenic marginal zone (pathway 2). CD27−IgA+ cells share low replication history and dominant Igλ and IgA2 use with gut lamina propria IgA+ B cells, suggesting their common origin from local germinal center-independent responses (pathway 3). Our findings shed light on human germinal center-dependent and -independent B-cell memory formation and provide new opportunities to study these processes in immunologic diseases.
Introduction
Antigen-specific memory formation after a primary infection contributes greatly to human health. Immunologic memory lies in long-lived T and B cells derived from the initial immune response. Precursor B cells develop from hematopoietic stem cells in the bone marrow and create a unique receptor by V(D)J recombination in their immunoglobulin (Ig) loci.1-3 After antigen recognition, mature B cells proliferate and can further optimize antigen-binding by the introduction of point mutations in the V(D)J exons of their Ig heavy and light chains (somatic hypermutations; SHMs) and the subsequent selection for high-affinity mutants.4 Furthermore, the antibody effector functions can be modified by changing the isotype of the IGH constant region from μ to α, δ, ϵ, or γ (Ig class-switch recombination; CSR).5 Both processes are mediated by activation-induced cytidine deaminase (AID), which preferentially targets specific DNA motifs.6,7
In addition to antigen recognition via the B-cell antigen receptor (BCR), B cells need a second signal to become activated.8 Activated T cells can provide such a signal via CD40L that interacts with CD40 on B cells. T cell–dependent B-cell responses are characterized by germinal center (GC) formation, extensive B-cell proliferation, affinity maturation, and Ig CSR.9 Thus, high-affinity memory B cells and Ig-producing plasma cells are formed. In addition, B cells can respond to T cell–independent (TI) antigens that either activate via the BCR and another (innate) receptor (TI-1) or via extensive cross-linking of the BCR because of the repetitive nature of the antigen (TI-2).10 TI responses are directed against blood-borne pathogens in the splenic marginal zone and in mucosal tissues (reviewed in Cerutti et al11 and Weill et al12 ).
A substantial fraction of B cells in blood of human subjects has experienced antigen and shows hallmarks of memory B cells: SHMs of rearranged Ig genes and fast recall responses to antigen.13 Initially, human memory B cells were identified based on the expression of CD27.14,15 IgA and IgG class-switched CD27+ B cells are derived from T cell–dependent responses in the GC and contain high loads of SHMs in their Ig genes.16-18 CD27+IgM+ B cells contain less SHMs but show molecular footprints of (early) GC generation.19 Interestingly, in contrast to CD27+IgM+IgD− “IgM-only” cells, CD27+IgM+IgD+ “natural effector” B cells are present in patients with CD40 or CD40L deficiency, indicating that at least part of this subset can be generated independently of T-cell help.17,20,21 Furthermore, natural effector B cells resemble splenic marginal zone B cells and have a limited replication history compared with GC B cells (both centroblasts and centrocytes) and CD27+IgD− memory B cells.17,18
More recently, CD27− IgG and IgA class-switched B cells have been described.22-24 CD27−IgG+ B cells contain fewer SHMs in their Ig genes and have increased IgG3 use compared with their CD27+ counterparts.22,23 Thus, 6 B-cell subsets have been described to contain genetic hallmarks of B-cell memory. This raises the question whether all these subsets show functional characteristics of memory B cells25 and whether the phenotypic diversity reflects functional diversity or an origin from different maturation pathways.
We performed detailed analyses on 6 phenotypically distinct memory B-cell subsets, which all seem to display an activated phenotype and molecular signs of antigen recognition. The comparative analyses of replication history, SHM, and CSR profiles of these subsets enabled us to trace their origins to 3 different germinal center-dependent and -independent maturation pathways.
Methods
Flow cytometric immunophenotyping and purification of B-cell subsets from human peripheral blood, tonsils, and colon
Peripheral blood, tonsil, and colon samples were obtained with informed consent following the Declaration of Helsinki and according to the guidelines of the Medical Ethics Committee of Erasmus MC and the Institutional Review Board of Weill Medical College of Cornell University.
Immunophenotyping and cell sorting details are provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Hematoxylin and eosin staining
Up to 30 000 cells from each sorted population were applied to poly-l-lysine–coated slides and stained with Diff-Quik staining set (Medion Diagnostics). Pictures were acquired on an Axioskop microscope using a Plan-NEOFLUAR 63/1.25 oil objective, MRc5 digital camera, and Axio Vision Release 4.8.1 software (Carl Zeiss).
CD40L-deficient patients
All 5 CD40L-deficient patients lacked expression of CD40L protein on activated T cells as shown after 5-hour stimulation with phorbol 12-myristate 13-acetate (Sigma-Aldrich) and calcium ionophore (Sigma-Aldrich). Mutations were detected by exon sequencing of the CD40L gene. Details of the patients are shown in supplemental Table 3.
Sequence analysis of complete IGH gene rearrangements and Ig switch regions
DNA was isolated from each sorted subset with the GenElute Mammalian Total DNA Miniprep kit, and RNA was isolated from Ig class-switched B-cell subsets using the GeneElute Mammalian Total RNA Miniprep kit (Sigma-Aldrich). Complete IGH gene rearrangements and hybrid switch regions were amplified and analyzed as described in supplemental Methods.
Replication history analysis using the KREC assay
The replication history of sorted B-cell subsets was determined with the Igκ-deleting recombination excision circles (KREC) assay as described previously.18 In brief, the amounts of coding and signal joints of the IGK-deleting rearrangement were measured by real-time quantitative-PCR in DNA from sorted B-cell populations on an ABI Prism 7000 sequence detection system (Applied Biosystems). Signal joints, but not coding joints are diluted 2-fold with every cell division.18 To measure the number of cell divisions undergone by each population, we calculated the ratio between the number of coding joints and signal joints. The previously established control cell line U698 DB01 (InVivoScribe) contains 1 coding and 1 signal joint per genome and was used to correct for minor differences in efficiency of both real-time quantitative-PCR assays.
IgκREHMA
The frequency of mutated IGK alleles was determined with the Igκ restriction enzyme hot-spot mutation assay (IgκREHMA) as described previously.18,26 In brief, PCR was performed on genomic DNA using a hexachlorofluorescein phosphoramidite (HEX)–coupled IGKV3-20 intron forward primer and two 5-carboxyfluorescein–coupled IGKJ reverse primers recognizing all 5 IGKJ genes. The PCR products were digested by the KpnI and Fnu4HI restriction enzymes and run on an ABI Prism 3130 XL genetic analyzer. Fnu4HI recognizes 2 adjacent sites in the unmutated gene product in the hot-spot region of IGKV-complementarity-determining region (CDR) 1. Unmutated gene products can therefore be visualized as 244- or 247-bp HEX-coupled fragments. KpnI cuts the gene product in FR2 downstream of the Fnu4HI sites, resulting in a 262-bp HEX-coupled mutated fragment. The unmutated B cell line CLL-1 was used as a positive control for complete digestion with Fnu4HI. The digests hardly contained undigested gene products of 481 bp, indicating complete digestion by KpnI.
Statistical analyses
Statistical analyses were performed with the Mann-Whitney U test, or χ2 test as indicated in details in figure legends. P values < .05 were considered statistically significant.
Results
Phenotypic characterization of memory B-cell subsets in healthy individuals
To study the diversity in the human B-cell compartment, we defined and purified 2 naive and 6 memory B-cell subsets (Figure 1A). Within the CD19+ B-cell compartment, we defined CD38hiCD24hi transitional B cells. CD38dimCD24dim B cells were subdivided based on the expression of IgM and CD27. Naive mature B cells were defined as CD27−IgM+. CD27+IgM+ B cells were separated into IgD+ “natural effector” B cells and IgD− “IgM-only” B cells. Finally, IgM-negative B cells were separated into 4 class-switched B cell populations based on the expression of IgA, IgG, and CD27.
Isolation and phenotypic characterization of peripheral blood memory B-cell subsets. (A) Gating strategy to identify 2 naive and 6 memory B-cell subsets based on expression of CD24, CD38, CD27, and IgH isotypes. (B) H&E staining of sorted subsets revealed a typical lymphocytic morphology with large nucleus (purple) and little cytoplasm (pink; ×63, original magnification; bars represent 5 μm). (C) All 6 memory B cell subsets showed up-regulation of CD80, CD180 and TACI as compared with naive B cells. Expression levels are shown in black and isotype controls as filled, gray histograms. Red lines indicate mode expression levels for each molecule on naive mature B cells.
Isolation and phenotypic characterization of peripheral blood memory B-cell subsets. (A) Gating strategy to identify 2 naive and 6 memory B-cell subsets based on expression of CD24, CD38, CD27, and IgH isotypes. (B) H&E staining of sorted subsets revealed a typical lymphocytic morphology with large nucleus (purple) and little cytoplasm (pink; ×63, original magnification; bars represent 5 μm). (C) All 6 memory B cell subsets showed up-regulation of CD80, CD180 and TACI as compared with naive B cells. Expression levels are shown in black and isotype controls as filled, gray histograms. Red lines indicate mode expression levels for each molecule on naive mature B cells.
All 8 purified subsets had a typical lymphocytic morphology with a large nucleus and little cytoplasm as observed after hematoxylin and eosin staining (Figure 1B). Furthermore, all 6 memory B-cell subsets showed an immunophenotype that was characteristic for activated cells; with increased expression of the B7 family member CD80, TLR-related CD180, and TNF receptor superfamily member TACI compared with naive B-cell subsets (Figure 1C).25,27 In addition, all B-cell subsets highly expressed BAFFR, and all memory B-cell subsets showed bimodal expression of inhibitory collagen receptor CD305 and were dimly positive for CD95 (data not shown).25,28 Thus, all 6 subsets we studied had the phenotype that was reported to be important for fast and powerful memory responses.
Ig repertoire selection in memory B-cell subsets
To study whether the memory B-cell subsets showed molecular signs of antibody selection, we sequenced IGH gene rearrangements from sorted fractions of healthy adult donors and compared these with naive B-cell subsets from adult blood as well as with GC B cells from childhood tonsils. We analyzed gene use for the most frequent IGHV subgroups: IGHV3 and IGHV4.29,30 All subsets showed diverse usage of IGHV3 subgroup genes with IGHV3-23, IGHV3-21, and IGHV3-30 predominating (Figure 2A). Naive mature B cells showed dominant use of the IGHV4-34 and IGHV4-59 genes (Figure 2B), probably resulting from increased recombination frequency because of highly efficient recombination signal sequences.31,32 Importantly, IGHV4-34 was hardly used in memory B-cell subsets, indicating selection against this inherently autoreactive gene.33,34
Selection against the IGHV4-34 gene and long IGH-CDR3s in all 6 memory B-cell subsets. (A) Frequencies of the most commonly used IGHV3 genes in cloned IGH gene rearrangements. Differences between each memory B-cell subset compared with naive mature B cells were statistically analyzed with the χ2 test. (B) Frequencies of the most commonly used IGHV4 genes in cloned IGH gene rearrangements. An arrow indicates IGHV4-34 gene use in naive mature B cells. Differences between each memory B-cell subset compared with naive mature B cells were statistically analyzed with the χ2 test. *P < .05, **P < .01. (C) IGH-CDR3 size distributions. All individual sizes are indicated for each subset as gray dots, with red lines representing the median values. The dashed and dotted lines represent median values for centroblasts (n = 67) and centrocytes (n = 55), respectively. Differences between each memory B-cell subset compared with naive mature B cells were statistically analyzed with the Mann-Whitney test. **P < .01, ***P < .001.
Selection against the IGHV4-34 gene and long IGH-CDR3s in all 6 memory B-cell subsets. (A) Frequencies of the most commonly used IGHV3 genes in cloned IGH gene rearrangements. Differences between each memory B-cell subset compared with naive mature B cells were statistically analyzed with the χ2 test. (B) Frequencies of the most commonly used IGHV4 genes in cloned IGH gene rearrangements. An arrow indicates IGHV4-34 gene use in naive mature B cells. Differences between each memory B-cell subset compared with naive mature B cells were statistically analyzed with the χ2 test. *P < .05, **P < .01. (C) IGH-CDR3 size distributions. All individual sizes are indicated for each subset as gray dots, with red lines representing the median values. The dashed and dotted lines represent median values for centroblasts (n = 67) and centrocytes (n = 55), respectively. Differences between each memory B-cell subset compared with naive mature B cells were statistically analyzed with the Mann-Whitney test. **P < .01, ***P < .001.
Of the 3 CDRs, the VDJ-junction encoded CDR3 region is the most dominant in establishment of antigen binding specificity. Long IGH-CDR3s are associated with auto- and polyreactivity.35 We observed diverse IGH-CDR3 sizes in transitional and naive mature B cells, with a median of 17 amino acids (Figure 2C). The median size was slightly reduced to 16 in both centroblasts and centrocytes. All memory B-cell subsets had significantly (P < .05) shorter IGH-CDR3s (median of 14-15 amino acids) compared with naive mature B cells. Thus, all 6 memory B-cell subsets showed comparable signs of Ig repertoire selection.
Distinct degrees of replication history and SHMs in memory B-cell subsets
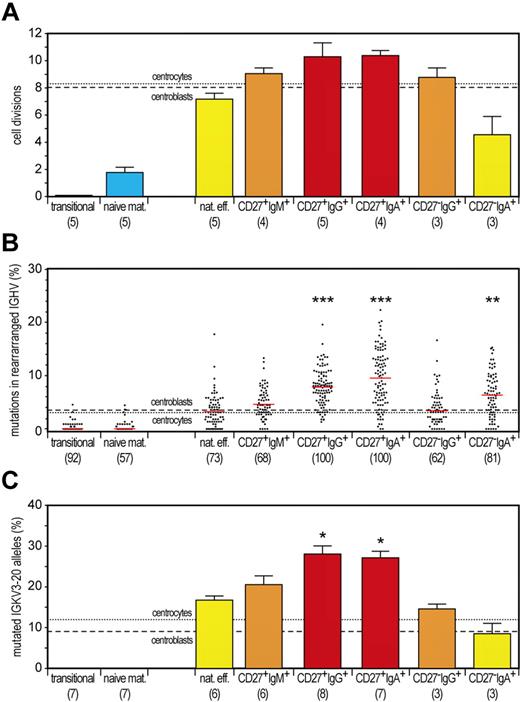
Typical hallmarks of memory B cells are extensive antigen-induced proliferation and SHMs. We showed previously that GC B cells in tonsils from young children have undergone ∼ 8 cell cycles, by calculating the ratio between genomic coding joints and signal joints on KREC of the IGK-deleting rearrangement.18 This replication history was similar in childhood CD27+IgD− B cells but clearly higher in adulthood CD27+IgD− cells, probably because of consecutive GC reactions. Proliferation of GC B cells was accompanied by SHMs in their Ig loci and further enrichment of mutated IGKV3-20 alleles in memory B cells, both in children and adults.18 We quantified the replication history, the frequency of mutated nucleotides in rearranged IGHV genes, and the frequency of mutated IGKV3-20 alleles in 2 naive and all 6 memory B-cell subsets. As shown before, transitional B cells did not undergo proliferation since their release from bone marrow, whereas naive mature B cells underwent ∼ 2 cell cycles in absence of SHMs (Figure 3).18 Conventional adult CD27+IgG+ and CD27+IgA+ B cells underwent the highest number of cell divisions (∼ 10) with high levels of SHMs. Both proliferation and SHM levels were clearly higher than in GC B cells from childhood tonsils. This might suggest additional proliferation and mutation in consecutive GC reactions.
Discrimination of GC-dependent and -independent B-cell maturation pathways based on quantitative analysis of the replication history and SHM levels. (A) Replication history of 2 naive and 6 memory B-cell subsets as measured with the KREC assay.18 Three different levels of extensive proliferation in memory B-cell subsets in contrast to naive B cells (blue) could be identified: lower than GC (yellow bars), similar to GC (orange bars) and increased compared with GC (red bars). Bars represent mean values with SEM. In the whole figure, dashed and dotted lines represent values for centroblasts and centrocytes, respectively. Differences between each memory B-cell subset compared with centrocytes were statistically analyzed with the Mann-Whitney test. (B) Frequency of mutated nucleotides in rearranged IGHV genes. All individual data points are shown as gray dots, with red lines indicating the median value. Differences between each memory B-cell subset compared with centrocytes were statistically analyzed with the Mann-Whitney test. **P < .01, ***P < .001. (C) Frequency of mutated IGKV3-20 genes as measured with the IgκREHMA assay. Bars represent mean values with SEM. Differences between each memory B-cell subset compared with centrocytes were statistically analyzed with the Mann-Whitney test. *P < .05.
Discrimination of GC-dependent and -independent B-cell maturation pathways based on quantitative analysis of the replication history and SHM levels. (A) Replication history of 2 naive and 6 memory B-cell subsets as measured with the KREC assay.18 Three different levels of extensive proliferation in memory B-cell subsets in contrast to naive B cells (blue) could be identified: lower than GC (yellow bars), similar to GC (orange bars) and increased compared with GC (red bars). Bars represent mean values with SEM. In the whole figure, dashed and dotted lines represent values for centroblasts and centrocytes, respectively. Differences between each memory B-cell subset compared with centrocytes were statistically analyzed with the Mann-Whitney test. (B) Frequency of mutated nucleotides in rearranged IGHV genes. All individual data points are shown as gray dots, with red lines indicating the median value. Differences between each memory B-cell subset compared with centrocytes were statistically analyzed with the Mann-Whitney test. **P < .01, ***P < .001. (C) Frequency of mutated IGKV3-20 genes as measured with the IgκREHMA assay. Bars represent mean values with SEM. Differences between each memory B-cell subset compared with centrocytes were statistically analyzed with the Mann-Whitney test. *P < .05.
IgM-only and CD27−IgG+ B cells underwent ∼ 9 cell divisions and had similar SHM levels in rearranged IGHV genes as GC B cells but increased frequencies of mutated IGKV3-20 alleles. The characteristics of both subsets suggest an origin from primary GC responses followed by selection for mutated IGKV3-20.
Finally, natural effector and CD27−IgA+ B-cell subsets showed less proliferation compared with GC B cells (Figure 3A). Natural effector B cells showed only 7 cell cycles, whereas the IGHV mutation loads were similar to GC B cells, and these cells were enriched for mutated IGKV3-20 alleles. These proliferation and SHM levels were clearly higher than those observed for natural effector cells in childhood tonsils.18 Still, these results indicate that a substantial fraction of this population had been generated independently from a GC. Finally, we observed only 4 cell divisions for CD27−IgA+ B cells. Interestingly, the IGHV gene mutation loads were increased as compared with GC B cells, although the frequency of mutated IGKV3-20 alleles was similar. These results indicate a GC-independent origin of CD27−IgA+ B cells but with high AID activity generating high SHM levels and IgA class switching. Still, these cells lacked selection for mutated IGKV3-20 alleles.
Targeting and selection of SHMs in rearranged IGHV genes
We analyzed type and targeting of SHMs in the memory B-cell subsets to obtain insight into the activity of AID, POLη, and UNG. Neither the SHM targeting nor the nucleotide substitution spectra and transition/transversion ratios were significantly different between the memory B-cell subsets and centrocytes (supplemental Table 1 and supplemental Figure 1). Furthermore, the targeting of specific nucleotides in motifs was largely similar between subsets (supplemental Table 2). Thus, we conclude that the differences in mutation frequencies did not result from altered AID, UNG, and Polη activities; rather, they probably reflect the duration of exposure to these enzymes.
Generally, a high ratio of replacement versus silent mutations (R/S) in IGHV-CDRs is regarded as a molecular sign of affinity maturation. Nevertheless, a clear cut-off value, which would reflect antigenic selection, remains difficult or even impossible to define.36 We found accumulation of replacement mutations in CDR1 and CDR2 of rearranged IGHV genes in all analyzed subsets (supplemental Figure 2). IGHV-CDR R/S ratios were similar between all memory B-cell subsets, ranging between 3.3 and 4.0, except for CD27−IgG+ B cells that had a slightly lower IGHV-CDR R/S ratio of 2.3 (supplemental Table 1).
Alignment of rearranged IGHV genes revealed the existence of recurrent amino acid changes (ie, the same amino acid replacement at the same position) in all except the natural effector, transitional, and naive mature B-cell subsets. In centrocytes, we identified a cluster of 5 sequences with identical VDJ gene use and closely similar if not identical IGH-CDR3s (always of identical length), pointing to their common ancestry. In addition to recurrent mutations, the sequences exhibited a different number and distribution of SHMs, indicating that the process of antigen-driven clonal expansion also was accompanied by intraclonal diversification.
IgG and IgA subclass distribution in class-switched memory B-cell subsets
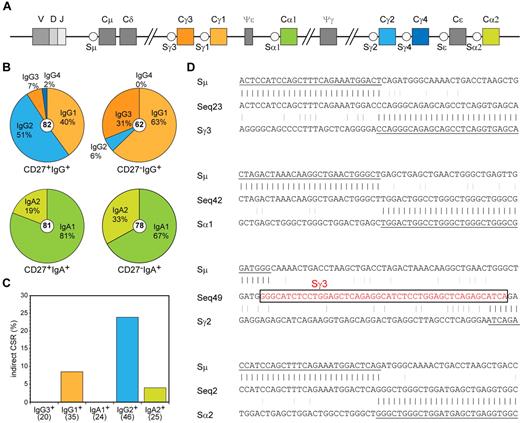
In addition to differential CD27 expression, both IgG+ and both IgA+ memory B-cell subsets varied in their replication history and SHM levels (Figure 3). This suggests different origins and functions for the CD27+ and CD27− B-cell subsets. Because the constant region of an antibody molecule is important for its function and the human IGH locus contains 4 IGHG and 2 IGHA constant genes (Figure 4A), we studied the Ig subclass use in sequenced IGH transcripts. We found a dominant use of IGHG2 (51%) and IGHG1 (40%) and low IGHG3 and IGHG4 in CD27+IgG+ cells (Figure 4B). In contrast, CD27−IgG+ cells showed a dominant use of IGHG1 (63%) and IGHG3 (31%) with little IGHG2 and no IGHG4.22,37 Thus, the CD27−IgG+ cells showed a dominant use of IGHM-proximal IGHG3 and IGHG1 regions (94%), whereas this was reduced to only 47% in CD27+IgG+ cells (P < .0001). Ig CSR to distal constant genes can occur indirectly via an IGHM-proximal gene. Analysis of hybrid switch regions (Sμ-Sγ2) in genomic DNA of sorted populations indeed revealed that 24% of junctions had remnants of Sγ3, Sγ1, or Sα1, whereas only 9% of Sμ-Sγ1 junctions had Sγ3 remnants (Figure 4C-D). Furthermore, the IGHV genes in IGHG2 and IGHG4 transcripts contained higher SHM loads than IGHG1 and IGHG3 (supplemental Figure 3A). The (indirect) switching to downstream IGHG genes accompanied by increased SHM frequencies suggests more prolonged AID activity in CD27+IgG+ cells, potentially reflecting multiple GC reactions.
Molecular analysis of Ig class switching in IgA+ and IgG+ memory B-cell subsets. (A) Schematic representation of the constant region of the human IGH locus. (B) Distribution of IgA and IgG receptor subclass use in IGH rearrangements of class-switched memory B-cell subsets. Total number of analyzed sequences is indicated in the center of each plot. Differences in the distribution were statistically analyzed with the χ2 test and were found significant for both CD27+IgG+ vs CD27−IgG+ (P < .0001) and CD27+IgA+ vs CD27−IgA+ (P < .05) B-cell subsets. (C) Frequency of Sμ-Sα and Sμ-Sγ rearrangements bearing remnants of indirect class switching. Number of analyzed sequences is given in brackets. (D) Examples of direct and sequential class switching; a piece of Sγ3 sequence in the Sμ-Sγ2 junction is indicated boxed in red font.
Molecular analysis of Ig class switching in IgA+ and IgG+ memory B-cell subsets. (A) Schematic representation of the constant region of the human IGH locus. (B) Distribution of IgA and IgG receptor subclass use in IGH rearrangements of class-switched memory B-cell subsets. Total number of analyzed sequences is indicated in the center of each plot. Differences in the distribution were statistically analyzed with the χ2 test and were found significant for both CD27+IgG+ vs CD27−IgG+ (P < .0001) and CD27+IgA+ vs CD27−IgA+ (P < .05) B-cell subsets. (C) Frequency of Sμ-Sα and Sμ-Sγ rearrangements bearing remnants of indirect class switching. Number of analyzed sequences is given in brackets. (D) Examples of direct and sequential class switching; a piece of Sγ3 sequence in the Sμ-Sγ2 junction is indicated boxed in red font.
The IgA+ memory B-cell subsets also showed differential subclass use: CD27−IgA+ memory B cells contained significantly more IGHA2 transcripts (33%) than CD27+IgA+ memory B cells (19%; Figure 4B; P < .05). Even though IGHA2 is the most downstream constant gene in the human IGH locus (Figure 4A), only 4% of hybrid Sμ-Sα2 regions contained remnants of more proximal S regions (Figure 4C-D), suggesting that most of the switching toward IGHA2 occurred directly from Sμ. No evidence for indirect class switching was found in Sμ-Sα hybrid switch regions. Furthermore, there was no difference in the mutation frequencies between IGHA1 and IGHA2 transcripts (supplemental Figure 3B). These results imply that switching toward IGHA2 occurs mainly directly from Sμ and the molecular differences between CD27+IgA+ and CD27−IgA− memory B cells most likely reflects their generation via separate response pathways, rather than consecutive GC reactions as observed for CD27−IgG+ versus CD27+IgG+ memory B cells.
T cell–independent generation of B-cell memory in CD40L-deficient patients
Replication history analyses indicated a GC-independent origin of natural effector and CD27−IgA+ B cells. To demonstrate that these subsets can be generated in the absence of the T-cell help, we analyzed their presence in 5 CD40L-deficient patients (supplemental Table 3). We found a clear population of natural effector B cells in CD40L-deficient patients, confirming previous observations that at least part of the blood natural effector B-cell population can be generated independently from T-cell help.17,20,21,38 Still, this subset was ∼ 3 times reduced in number compared with age-matched healthy controls (Figure 5), highlighting the fact that in healthy controls a major part of this subset has a germinal center origin. More importantly, blood of CD40L-deficient patients also contained CD27−IgA+ memory B cells, and their numbers were similar compared with healthy controls (Figure 5). Thus, in addition to natural effector cells, T cell–independent humoral responses in human can generate IgA class-switched memory B cells.
GC-independent generation of natural effector and CD27−IgA+ memory B cells. (A) Memory B-cell subset distribution was analyzed in 5 CD40L-deficient patients (age, 1-13 years) and 50 healthy controls (age, 1-5 years). Representative FACS plots of B-cell subsets. (B) Absolute cell numbers of 6 memory B-cell subsets. Bars represent mean values with SEM. Statistical significance was calculated with the Mann-Whitney test. * P < .05, **P < .01, ***P < .001.
GC-independent generation of natural effector and CD27−IgA+ memory B cells. (A) Memory B-cell subset distribution was analyzed in 5 CD40L-deficient patients (age, 1-13 years) and 50 healthy controls (age, 1-5 years). Representative FACS plots of B-cell subsets. (B) Absolute cell numbers of 6 memory B-cell subsets. Bars represent mean values with SEM. Statistical significance was calculated with the Mann-Whitney test. * P < .05, **P < .01, ***P < .001.
CD27−IgA+ memory B cells resemble colon lamina propria IgA+ B cells
T cell–independent responses have been demonstrated to generate IgA-producing B cells in the lamina propria of human gut.39,40 Furthermore, these IgA+ B cells showed predominant use of IGHA2.41 These similarities with blood CD27−IgA+ memory B cells encouraged us to study whether these cells had been generated in similar responses. First, we analyzed the replication history of IgA2+ B cells isolated from colon lamina propria. Similar to CD27−IgA+ B cells, these cells had proliferated less than GC B cells in childhood tonsils and significantly less than GC-derived CD27+IgA+ memory B cells in adult blood (Figure 6A). In addition, because it was suggested previously that a broad Igλ repertoire may be beneficial for responses in the human gastrointestinal tract,42 we analyzed the κ/λ light chain isotype ratios of blood B-cell subsets by flow cytometry. We found a high frequency of Igλ+ cells (80%) within the CD27−IgA+ B-cell subset compared with both CD27+IgA+ cells (55%) and naive mature B cells (45%; Figure 6B). Sequence analysis of IGLV-IGLJ rearrangements revealed fewer mutations in CD27−IgA+ than in CD27+IgA+ memory B cells, despite similar IGLV and IGLJ gene use and IGL-CDR3 size and composition (Figure 6C; supplemental Table 4). The molecular similarities between CD27−IgA+ B cells and gut lamina propria IgA-producing B cells suggest a com-mon origin of these cells from local responses in the gastrointestinal tract.
CD27−IgA+ memory B cells resemble colon lamina propria IgA+ B cells. (A) Replication history in naive mature, IgA+ memory B-cell subsets and CD19+IgA2+ B cells isolated from human colon lamina propria as measured with the KREC assay. Bars represent mean values with SEM. Statistical significance was calculated with the Mann-Whitney test. (B) Igκ and Igλ isotype distribution of naive mature and IgA+ memory B cell subsets as determined with flow cytometric analysis. (C) Frequency of mutated nucleotides in rearranged IGLV gene segments. All individual data points are showed as gray dots, with red lines indicating the median value. Statistical significance was calculated with the Mann-Whitney test. **P < .01.
CD27−IgA+ memory B cells resemble colon lamina propria IgA+ B cells. (A) Replication history in naive mature, IgA+ memory B-cell subsets and CD19+IgA2+ B cells isolated from human colon lamina propria as measured with the KREC assay. Bars represent mean values with SEM. Statistical significance was calculated with the Mann-Whitney test. (B) Igκ and Igλ isotype distribution of naive mature and IgA+ memory B cell subsets as determined with flow cytometric analysis. (C) Frequency of mutated nucleotides in rearranged IGLV gene segments. All individual data points are showed as gray dots, with red lines indicating the median value. Statistical significance was calculated with the Mann-Whitney test. **P < .01.
Model of memory B-cell generation from 3 distinct pathways
Here, we demonstrate by molecular analysis of Ig genes that 6 distinct memory B-cell subsets can be identified based on their IgH isotype and expression of CD27. To recapitulate our findings, we propose a modified scheme of memory B-cell generation (Figure 7): CD27−IgA+ B cells and natural effector B cells (at least in part) are derived from local and systemic GC-independent responses, respectively; CD27−IgG+ and CD27+IgM+ B cells are derived from primary GC responses and CD27+IgG+ and CD27+IgA+ B cells (at least in part) from secondary GC responses.
Model of human memory B-cell generation from GC-dependent and -independent pathways. Six purified memory B-cell subsets showed differential levels of proliferation and BCR maturation. Ig class-switching profiles and immunophenotyping of blood of CD40L-deficient patients supported delineation of these 6 subsets from T cell-dependent and -independent maturation pathways. CD27−IgA+ and natural effector B cells can be derived independently from T-cell help, probably locally in the gastrointestinal tract and from systemic responses in splenic marginal zone, respectively. The molecular profiles of CD27−IgG+ and CD27+IgM+ memory B cells resembled those of primary GC cells, whereas CD27+IgG+ and CD27+IgA+ memory B cells has increased proliferation and SHM levels suggestive of further maturation in consecutive GC response.
Model of human memory B-cell generation from GC-dependent and -independent pathways. Six purified memory B-cell subsets showed differential levels of proliferation and BCR maturation. Ig class-switching profiles and immunophenotyping of blood of CD40L-deficient patients supported delineation of these 6 subsets from T cell-dependent and -independent maturation pathways. CD27−IgA+ and natural effector B cells can be derived independently from T-cell help, probably locally in the gastrointestinal tract and from systemic responses in splenic marginal zone, respectively. The molecular profiles of CD27−IgG+ and CD27+IgM+ memory B cells resembled those of primary GC cells, whereas CD27+IgG+ and CD27+IgA+ memory B cells has increased proliferation and SHM levels suggestive of further maturation in consecutive GC response.
Discussion
In this study, we set out to relate distinct memory B-cell subsets to the diverse humoral response types that have been documented in the literature. We defined 6 memory B-cell subsets with phenotypic and molecular signs of antigen encounter. Detailed comparative analysis of their Ig genes, comparison with tissue-derived B-cell subsets, and analysis of memory B-cell subsets in CD40L-deficient patients allowed us to distinguish 3 unique maturation pathways: GC-independent local and systemic responses and GC-dependent responses. Furthermore, we delineated primary and consecutive phases of GC responses.
The CD27+IgA+ and CD27+IgG+ subsets are generally regarded as true B-cell memory.25 Whereas this qualification is somewhat controversial for CD27+IgM+ subsets and CD27− class-switched subsets, our results strongly support these to be true memory B cells based on the (1) high expression of activation and costimulatory molecules; (2) selection against inherently autoreactive VH domain characteristics; (3) extensive replication history compared with naive B cells; and (4) SHM profiles of Ig heavy and light variable genes with high R/S ratios in IGH-CDRs. Despite these common features of B-cell memory, we found clear quantitative differences in proliferation, SHM, and CSR processes among these subsets. We conclude that these differences reflect different origins and maturation pathways before becoming memory B cells. Consequently, these differences justify dividing the memory B-cell compartment into subsets.
Of the 6 memory B-cell subsets, the CD27+IgG+ and CD27+IgA+ B cells had the highest degrees of proliferation and SHMs. Interestingly, these levels were higher than those of GC B cells from childhood tonsils. We previously observed increased proliferation and SHMs in CD27+IgD− cells from adults compared with children and concluded that in adults at least part of these cells had undergone additional immune responses on secondary or tertiary antigen encounter.18 Our current results showed similar additional proliferation and SHMs for both CD27+IgA+ and CD27+IgG+ B cells. Furthermore, the increased proliferation was accompanied by increased use of distally located IGHG2 and IGHG4 genes with signs of indirect CSR. Thus, these results support the concept that at least part of the CD27+IgA+ and CD27+IgG+ B-cell subsets in healthy adults has undergone multiple immune responses.
Interestingly, the IGHV gene mutation frequency was clearly higher in CD27+IgA+ compared with CD27+IgG+ B cells. Because the targeting of mutations was similar, AID and UNG activities seemed unaffected. Rather, CD27+IgA+ B cells might have experienced prolonged AID and UNG activities. Because IgA class switching mostly occurs in mucosa-associated lymphoid tissues, this difference might reflect the location of the immune response. Still, despite these higher mutation loads, we found no differences in replacement mutation patterns in IGHV genes or the frequency of mutated IGK alleles, suggesting similar selection mechanisms for both CD27+IgA+ and CD27+IgG+ B cells.
We conclude that IgM-only and CD27−IgG+ B cells are derived from primary GC responses. This was based on their highly similar replication history and IGHV gene mutation loads compared with GC B cells from childhood tonsils and is further supported by the dominant use (> 90%) of the IGHM-proximal IGHG1 and IGHG3 genes in CD27−IgG+ B cells. In contrast to IGHV gene mutation loads, the frequencies of mutated IGKV alleles were increased in both subsets compared with GC B cells. We previously found this increased frequency in tonsillar CD27+IgD− memory B cells.18 Because this occurred in the absence of additional proliferation, it probably reflects positive selection for the mutated hot-spot in memory B cells rather than additional mutations.
IgM responses are initiated early in primary infection. Dogan et al43 described that following primary immunization of mice, IgM+ memory B cells were formed that on secondary challenge could class switch toward IgG1+ cells. Furthermore, clonally related IgM+ and IgG+ B cells were found in human GCs and peripheral blood.19,44 Thus, compared with CD27+IgA+ and CD27+IgG+ memory B cells, CD27+IgM+ memory B cells are early GC emigrants that did not undergo class switching.45 Still, 2 issues have hampered proper studies on IgM+ memory B cells in recent years. First, the CD27+IgM+IgD− and CD27+IgM+IgD+ subsets have not always been separated, despite evidence that only the CD27+IgM+IgD+ subset contains cells that have been generated independently from GCs (Figure 5B).17,21 Second, often the CD27+IgD− population is not further subdivided. As a consequence, this is a mixed population of Ig class-switched and IgM+ memory B cells. Our results demonstrate that this has no major implications, because these subsets all seem GC dependent. However, it should be noted that the CD27+IgD− subsets contain a substantial fraction of IgM+ memory B cells, particularly in young children; therefore, it should be avoided to address these as “Ig class-switched memory.”
The low SHM loads in CD27−IgG+ B cells compared with CD27+IgG+ B cells have lead to speculations on the origin of these cells: from T cell-independent responses or first wave from a GC reaction.22,23 We found that the replication history and SHM levels of CD27−IgG+ B cells highly resemble GC B cells. Furthermore, CD27−IgG+ B cells were hardly detectable in CD40L-deficient patients and they have dominant use of IgM-proximal IgG3 and IgG1 subclasses. Thus, we conclude that similarly to CD27+IgM+ cells, CD27−IgG+ cells are derived from primary GC-dependent responses.
Several studies have shown an expansion of both CD27+IgM+IgD− and CD27−IgG+ memory B cells in autoimmune diseases.17,22,23 Interestingly, CD27−IgG+ B cells dominantly use IgG1 and IgG3, which are potent activators of the complement system and inducers of antibody-dependent cell-mediated cytotoxicity.46 Thus, our observations of the different IgG subclass use in CD27+IgG+ versus CD27−IgG+ B cells suggest a potential role of CD27−IgG+ cells in autoimmunity. Still, additional studies need to address whether many CD27−IgG+ B cells carry an autoreactive BCR or whether other mechanisms result in deregulation of CD27−IgG+ B cells in patients with an autoimmune disease.
In contrast to the other memory B-cell subsets, natural effector and CD27−IgA+ B cells showed limited proliferation compared with GC B cells and were present in CD40L-deficient patients. Thus, we concluded that these cells can be generated independent from T-cell help. It is debated whether CD27+IgM+IgD+ natural effector B cells in healthy adults are generated from germinal center responses or independently of T-cell help in the splenic marginal zone.17,19-21 We describe reduced replication history and SHM levels in natural effector B cells compared with IgM-only memory B cells. Because IgM-only memory B cells highly resemble germinal center B cells on the molecular level, we conclude that in healthy adults part of the natural effector B cells can be generated outside of a GC. Thus, the natural effector B-cell subset in healthy individuals is probably a mixed population of GC-derived and splenic marginal zone-derived memory B cells. Interestingly, a recent study described the presence of CD27+CD43+CD20+ B1 cells in umbilical cord blood and in adult peripheral blood.47 It is possible that the T cell–independent characteristics ascribed to CD27+IgM+IgD+ natural effector B cells are specific for the CD43+ fraction. This should be further investigated.
The CD27−IgA+ memory B-cell subset was the smallest population we studied and, to our knowledge, we showed for the first time that these cells can be derived independent from T-cell help. TI IgA responses have been observed both in human and mouse, both systemically in the splenic marginal zone and locally in the gastrointestinal system.40,48,49 Potential mediators of CD40-independent IgA CSR are BAFF and APRIL.39 Because blood CD27−IgA+ B cells and gut lamina propria IgA-producing B cells were highly similar in their limited replication history, and dominant IgA2 and Igλ light chain isotypes, we conclude that these cells have been generated in similar responses. Although the anatomic location of TI CSR toward IgA in human gut remains controversial,40,50 on the basis of our findings, we can state that CD27−IgA+ memory B cells resemble IgA+ cells from the gut lamina propria and seem to be a blood counterpart of this IgA-producing population. Even though analysis of the memory B-cell compartment showed that CD27−IgA+ B cells seem completely TI, we cannot exclude that in physiologic conditions a minor fraction of CD27−IgA+ B cells is generated in a primary immune response analogous to CD27−IgG+ B cells.
The human memory B-cell compartment is more complex than originally thought and actually consists of diverse subsets that have originated from functionally distinct responses. Interestingly, differential expression of CD27 was the key to identification of the diverse subsets. The function of CD27 on B cells remains unclear. CD27-CD70 interactions can trigger plasma cell differentiation and provide negative feedback signals. Thus, CD27+ and CD27− memory B cells might function differently. Still, the similar up-regulation of many other costimulatory molecules on these cells might compensate for the lack of CD27. Alternatively, the differential CD27 expression might reflect the different types of responses in which the cells have been generated and thus represents a useful marker to discriminate between these subsets.
Different levels of memory B-cell responses seem to reflect the phylogenetic evolution of the immune system from local TI responses, via systemic TI responses to most advanced T cell–dependent responses in the GC. These different origins suggest unique physiologic functions in protection against pathogens.
In this study, we dissected the human memory B-cell compartment into 6 distinct subsets. Molecular analysis of these memory B cells in healthy controls and comparison with memory B cells from CD40L-deficient patients and colon lamina propria B cells enabled us to delineate their origin from 3 different maturation pathways: local and systemic TI responses and primary or secondary GC responses. Because these B-cell subsets are present in blood, our study provides new opportunities to analyze these processes in patients with (auto)inflammatory conditions, B-cell immunodeficiencies, and nodal and extranodal B-cell malignancies.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to D. van den Heuvel, E. F. E. de Haas, and S. J. W. Bartol for technical support; to S. de Bruin-Versteeg for assistance with preparing the figures; and to Dr N. S. Longo for assistance with JOINSOLVER (http://joinsolver.niams.nih.gov) analysis.
This work was supported by a grant from the Erasmus University Rotterdam (EUR-Fellowship to M.C.v.Z.).
National Institutes of Health
Authorship
Contribution: M.A.B, J.J.M.v.D., and M.C.v.Z. designed the experiments; G.J.A.D., K.S., A.C., and M.v.d.B. provided conceptual advice; M.A.B. and C.G.-W. performed and analyzed most of the experiments; G.J.A.D., V.B., and K.S. contributed to data analyses; G.J.A.D., B.H., A.C., K.B, J.F.L., and M.v.d.B. provided material necessary for performing experiments; M.A.B. and M.C.v.Z. wrote the manuscript; and G.J.A.D., V.B., C.G.-W., K.S., A.C., M.v.d.B., and J.J.M.v.D. commented on the manuscript.
Conflict-of-interest disclosure: J.J.M.v.D. is the inventor of the KREC assay, which has been patented (PCT/NL 2005/00761; priority date October 25, 2004) and licensed to In Vivo Scribe Technologies; revenues of the patent go to Erasmus MC. The remaining authors declare no competing financial interests.
Correspondence: Menno C. van Zelm, Department of Immunology, Unit Molecular Immunology, Erasmus MC, University Medical Center, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands; e-mail: m.vanzelm@erasmusmc.nl.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal