Filamins are crucial for human development and they may play a role in the regulation of megakaryocyte maturation. Still, little is known about the molecular mechanisms involved in platelet production from megakaryocytes and, consequently, how those mechanisms impact the pathogenesis of platelet disorders. In this issue of Blood, Jurak Begonja and colleagues describe, for the first time, how the absence of filamin A can impair platelet production and survival in the peripheral circulation in a mouse model.1
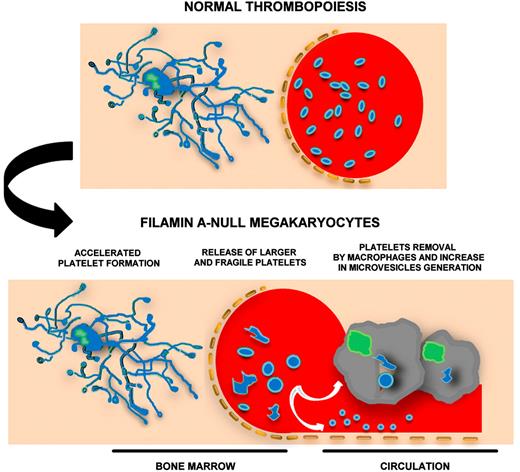
Filamin A-null megakaryocytes rapidly release fragile and giant platelets that microvesiculate and are promptly removed from circulation by macrophages.
Filamin A-null megakaryocytes rapidly release fragile and giant platelets that microvesiculate and are promptly removed from circulation by macrophages.
Filamins combine cell adhesion and signaling systems serving as a scaffold for proteins and linking actin networks to cell membranes.2 These diverse and versatile functions suggest that FLNA and FLNB mutations could be the origin of several human diseases. Indeed, null or specific mutations in FLNA and FLNB determine a wide range of developmental malformation of the brain, bones, and heart in humans.3 Filamin A is the most abundant and widely expressed isoform. In blood cells, particularly erythrocytes and platelets, filamin A plays an important role in the mechanical stabilization of the plasma membrane to protect cells against shear and distortion forces in the circulatory system.4 Further, in platelets, filamin A anchors the adhesion glycoprotein (GP) Ib-IX-V receptor to actin cytoskeleton promoting platelet adhesion to von Willebrand factor and downstream signal transduction.5
Mutations on chromosome Xq28, which encodes the actin-binding protein filamin A, cause X-linked bilateral periventricular nodular eterotopia (BPNH), characterized by several malformations including vascular defects and hemorrhage associated or not with thrombocytopenia.3 More than 50 mutations have been reported for FLNA in BPNH. Recently, the p.V528M variant was found in a patient with suspected X-linked macrothrombocytopenia and classified as functional polymorphism because no associations were found with neither BPNH nor macrothrombocytopenia.6 Interestingly, in a previous paper, the Falet group has shown that Filamin AloxP/y GATA1-Cre mice, which lack filamin A in platelets, present macrothrombocytopenia with decreased expression and functional impairment of GPIbα.7
In this issue of Blood, Jurak Begonja et al propose a multifaceted mechanism that leads to macrothrombocytopenia in the absence of filamin A in a PF4-Cre transgenic mouse model.1 Megakaryocytes, lacking filamin A, differentiate normally, but rapidly release fragile platelets that are cleared by macrophages in the circulation (see figure). Filamin AloxP PF4-Cre mice had a severe macrothrombocytopenia that was partially rescued by removing macrophages with intravenous injection of clodronate-encapsulated liposomes. As a consequence of thrombocytopenia, increased numbers of megakaryocytes were observed in both bone marrow and spleen of Filamin AloxP PF4-Cre mice. Despite normal differentiation, fetal liver–derived filamin A-null megakaryocytes extended fewer proplatelets, in vitro, that rapidly released larger platelets than controls, as shown by flow cytometry and time-lapse video microscopy analysis.
The macrothrombocytopenia of Filamin AloxP PF4-Cre mice resembles that observed in Bernard-Soulier Syndrome (BSS). Although different studies on mouse models and human patients have been conducted, the exact mechanisms that lead to BSS are unknown. In addition, the question about the role played by the extracellular and the cytoplasmatic GPIbα domains on platelet formation is still open.8,9 From this perspective, the article by Jurak Begonja et al sheds new light on the role played by the GPIb-IX-V complex in megakaryocyte development. There are several new clues: (1) A time course analysis indicates that expression of the von Willebrand factor receptor and filamin A starts in immature megakaryocytes and increases just before proplatelet extension. (2) The association of GPIb to actin cytoskeleton occurs only in mature megakaryocytes and is filamin A–dependent, as in platelets. (3) GPIbα expression on filamin A-null megakaryocyte membranes is similar to controls, in contrast with filamin A-null platelets that have decreased GPIbα surface levels. All together, these data point out the importance of a correct association between the actin-rich membrane skeleton and the plasma membrane to platelet production. It looks like macrothrombocytopenia is always accompanied by a defect in some cytoskeleton components (ie, tubulin, myosin IIA, filamin A). Megakaryocytes have to be in “perfect” shape to release functional platelets.
The increase of the microtubular mass in giant platelets may be ascribed to the incapacity of pro-platelets to undergo the final step that occurs in circulation and leads to production of correct numbers of normal-sized platelets. Alternatively, giant platelets may stay giant to have a thicker microtubule coil as a sort of protection of their fragile structure. Finally, filamin A-null platelets undergo microvesiculation while they lose von Willebrand factor receptor components on the membrane surface. This may be explained by the fact that, in the absence of filamin A, ADAM17 and MMP9 metalloproteinases are more expressed and, in turn, GPIbα is more cleaved. Furthermore, the formation of annexin-V+ microvesicles was strongly associated with the activity of the protease calpain. Thus, filamin A acts to maintain the synergy between cellular mechanics and signaling systems that is required to regulate the production of structured and functional platelets.
Beside GPIbα and von Willebrand factor, filamin A interacts with several proteins that regulate cell adhesion, including β1 integrin and several protein kinases.2 Megakaryocyte adhesion and migration on extracellular matrices are key factors in the regulation of platelet formation by the bone marrow environment. Interestingly, Falet et al recently demonstrated that filamin A is required in signaling by direct interaction with Syk in platelet activation through the immunoreceptor tyrosine-based activation motif (ITAM)– and ITAM-like–mediated signal receptors GPVI and C-type lectin-like receptor 2.7 This observation, together with the knowledge that filamin A regulates cell adhesion strength, raises a variety of very interesting questions about the role of filamin A in the interaction of megakaryocytes with bone marrow matrices, such as collagen I. Little is known about the transmission of force between hemopoietic stem cells and their niche, and the question on how biochemical and biomechanical signals cooperate to generate force and, ultimately, to regulate megakaryocyte fate, is still open.
The study by Jurak Begonja et al clearly demonstrates that, despite their tight affinity, megakaryocytes do not always behave like platelets and that platelet formation is the consequence of a variegated sequence of events. Thus, only the fine-tuning of the different elements of the orchestra will lead to the release of functional platelets.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal