Abstract
Hyperhomocysteinemia (HHcy) is associated with endothelial dysfunction (ED), but the mechanism is largely unknown. In this study, we investigated the role and mechanism of HHcy-induced ED in microvasculature in our newly established mouse model of severe HHcy (plasma total homocysteine, 169.5μM). We found that severe HHcy impaired nitric oxide (NO)– and endothelium-derived hyperpolarizing factor (EDHF)–mediated, endothelium-dependent relaxations of small mesenteric arteries (SMAs). Endothelium-independent and prostacyclin-mediated endothelium-dependent relaxations were not changed. A nonselective Ca2+-activated potassium channel (KCa) inhibitor completely blocked EDHF-mediated relaxation. Selective blockers for small-conductance KCa (SK) or intermediate-conductance KCa (IK) failed to inhibit EDHF-mediated relaxation in HHcy mice. HHcy increased the levels of SK3 and IK1 protein, superoxide (O2−), and 3-nitrotyrosine in the endothelium of SMAs. Preincubation with antioxidants and peroxynitrite (ONOO−) inhibitors improved endothelium-dependent and EDHF-mediated relaxations and decreased O2− production in SMAs from HHcy mice. Further, EDHF-mediated relaxation was inhibited by ONOO− and prevented by catalase in the control mice. Finally, L-homocysteine stimulated O2− production, which was reversed by antioxidants, and increased SK/IK protein levels and tyrosine nitration in cultured human cardiac microvascular endothelial cells. Our results suggest that HHcy impairs EDHF relaxation in SMAs by inhibiting SK/IK activities via oxidation- and tyrosine nitration–related mechanisms.
Introduction
Hyperhomocysteinemia (HHcy) is a potent risk factor for cardiovascular disease and is associated with impaired endothelium-dependent vasodilation in both experimental animals1-4 and humans.5,6 Endothelium plays a key role in the control of vasomotor tone and organ perfusion and contributes to the regulation of arterial blood pressure by releasing vasodilator substances such as nitric oxide (NO), prostacyclin (PGI2), and endothelium-derived hyperpolarizing factor (EDHF), and vasoconstrictor substances, such as angiotensin II, endothelin-1, thromboxane A2, and prostaglandin H2, in response to pathophysiological stimulation.4,7 Endothelial dysfunction (ED), which is characterized by an impairment of endothelium-dependent vasodilatation, is an early event in the development of cardiovascular disease. It is generally agreed that NO predominantly controls large arterial relaxation, whereas EDHF primarily controls small arterial relaxation and is more important when vessel diameter decreases.8-10 We and others reported that severe HHcy impairs endothelium-dependent relaxation in mouse aortas1 and EDHF-mediated vascular relaxation in rat renal arteries,3,11 mouse cremaster arteries,1 and mouse mesenteric arteries.12 However, the molecular mechanism through which HHcy inhibits EDHF-mediated relaxation has not been explored.
The nature of EDHF is not well understood. It is defined as endothelium-dependent relaxation that is mediated by NO- and PGI2-independent mechanisms.13 It has been suggested that EDHF-mediated responses are initiated by the activation of endothelial Ca2+-activated potassium channels (KCas) with resultant hyperpolarization of endothelial cells. Hyperpolarization of endothelium is then transferred to vascular smooth muscle cells (VSMCs) by synthesizing or generating signals capable of diffusing through membranes or myoendothelial gap junctions. In VSMCs, EDHF activates KCas and voltage-dependent Ca2+ channels causing hyperpolarization and relaxation.10 The KCa family consists of small conductance KCas (SKs, including SK1, SK2, and SK3), intermediate conductance KCas (IK1s), and large-conductance KCas (BKs). Current understanding supports the concept that EDHF signaling is mainly initiated by activation of SKs and IKs, especially SK3 and IK1, which are located in the endothelium of small arteries.14
In the present study, we examined the role of HHcy on EDHF-mediated vascular relaxation in small mesenteric arteries (SMAs) isolated from our newly developed mouse model of HHcy, in which the mouse cystathionine β-synthase (Cbs) gene is deficient and a zinc-inducible human CBS transgene is introduced to circumvent the neonatal lethality of Cbs deficiency (Tg-hCBS Cbs−/−). The Tg-hCBS Cbs−/− mice developed severe HHcy (plasma total homocysteine = 169.5 ± 3.5μM), and provided a consistent and sensitive biochemical environment that permitted detailed mechanistic assessment of endothelial function.
Methods
Transgenic mice and homocysteine (Hcy) measurement
The Tg-hCBS Cbs−/− mice were created as described previously.15,16 Animals were born to Tg-hCBS Cbs−/+ mothers on drinking water containing 25mM ZnCl2 to induce transgene expression. ZnCl2 was withdrawn after weaning at 1 month of age. For all experiments, control mice were sibling transgene positive mice that were in either Cbs−/+ or Cbs+/+ background. The Cbs−/+ and Cbs+/+ mice in the control groups have similar plasma total homocysteine levels.15,17 Animals were fed standard rodent chow containing 0.43% methionine (TD 2018SX; Harlen Teklad). Plasma from 4-hour-fasted mice was collected into 4mM EDTA-coated tubes at the end of each experiment. A total of 50 μL of plasma was then analyzed for Hcy levels using a Biochrom 30 amino acid analyzer as described previously.15,16 Mouse protocols were approved by the Temple University and Fox Chase Cancer Center Institutional Animal Care and Use Committees.
SMA preparation
Tg-hCBS Cbs−/− and control mice at the age of 8-10 months were killed by stunning and exsanguination under anesthesia and the mesenteric bed removed. SMAs (2-mm segments of the second-order branch of the superior mesenteric artery, 150- to 200-μm diameter) was dissected free of fat and connective tissue and mounted in Mulvany-style isometric myographs (DMT) for vessel reactivity assessment. Vessels were maintained at 37°C in physiologic Krebs buffer of the following composition: NaCl, 120mM; NaHCO3, 25mM; KCl, 4.8mM; NaH2PO4, 1.2mM; MgSO4, 1.2mM; dextrose, 11.0mM; CaCl2, 1.8mM aerated with 95% O2 and 5% CO2 (pH = 7.4). Data were captured using a Power Lab data acquisition system (AD Instruments). The rings were equilibrated for 60 minutes at 37°C with a resting tension of 1.5mN and then assessed for vascular function.
Vascular contractile responses
After an equilibration period, SMA rings were exposed to 120mM KCl until reproducible and maximal depolarization-induced contractions were achieved. After a second round of washing and equilibration with Krebs, vascular contractile responses to cumulative additions of phenylephrine (PE; 10nM-33μM), which causes α1-adrenoceptor–induced contractions, were determined.
Vascular relaxation responses
The presence of intact endothelium in the vascular preparations was confirmed by observing the relaxation response to 1μM acetylcholine (ACH) in rings precontracted with 1μM PE, as described previously.18 Endothelium-dependent relaxation responses to cumulative concentrations of ACH (10nM-33μM) and endothelium-independent relaxation responses to sodium nitroprusside (SNP; 1nM-10μM) in rings precontracted with PE (1μM) were determined. PGI2-mediated endothelium-dependent vascular relaxation was recorded in the presence of NG-nitro-L-arginine methyl ester hydrochloride (L-NAME; 100μM), a NOS inhibitor, and tetraethylammonium bromide (TEA; 1mM), a nonselective KCa blocker, for 30 minutes to inhibit NO formation and EDHF, respectively. NO-mediated endothelium-dependent relaxation was examined in the presence of a combination of indomethacin (INDO; 10μM), a nonselective cyclooxygenase (COX) inhibitor that blocks the formation of PGI2 and TEA. EDHF-mediated endothelium-dependent relaxation (or EDHF-mediated relaxation) was determined in the presence of L-NAME and INDO.
EDHF-mediated relaxation
To determine the mechanisms underlying EDHF-mediated relaxation, SMAs were incubated with TEA (1mM); apamin, a selective SK inhibitor (1μM); or Tram34, a selective IK inhibitor (1μM) for 30 minutes in the presence of INDO and L-NAME, inhibitors of PGI2 and NO production, respectively. KCa-linked EDHF-mediated maximal relaxation was calculated as: ([EDHF-mediated maximal relaxation]-[EDHF-mediated maximal relaxation in the presence of KCa inhibitor])/([EDHF-mediated maximal relaxation]-[EDHF-mediated maximal relaxation in the presence of TEA]) × 100 in the control mice. Values of KCa-controlled EDHF-mediated relaxation in control mice were defined as 100%.
Mechanistic assessments for impaired endothelial-dependent and EDHF-mediated relaxation
To access the biochemical mechanism of HHcy-induced ED, SMAs were preincubated with various pharmacologic inhibitors for specific signaling pathways. These included the antioxidants polyethylene glycol–superoxide dismutase (PEG-SOD; 150 U/mL), PEG-catalase (PEG-Cat; 150 U/mL), and Tempol (1mM), a scavenger of O2−, and the peroxynitrite (ONOO−) scavengers ebselen (10μM) or uric acid (100μM) for 1 hour before testing endothelium- or EDHF-dependent relaxation.
NS309, an IK and SK opener,19 was used to determine the role of IK and SK in ONOO−- and HHcy-impaired EDHF-mediated relaxation by incubating SMAs from control mice pretreated with 100μM ONOO− for 30 minutes or from Tg-hCBS Cbs−/− mice pretreated with 1μM NS309 for 30 minutes. The effect of ONOO− on EDHF-mediated vascular relaxation in response to ACH was examined by pretreating SMAs of control mice with 100μM ONOO− for 0.5 hours before testing EDHF-dependent relaxation or testing relaxation to cumulative additions of NS309 (10nM-10μM).
Vascular and endothelial cell O2− production
Fresh SMA segments or cross-sections (30-μm) were equilibrated for 30 minutes at 37°C in Krebs buffer, and then incubated in fresh buffer containing dihydroethidium (DHE; 3μM) for 30 minutes in the dark. Cultured human cardiac microvascular endothelial cells (HCMECs) were treated with PEG-SOD (150 U/mL) or Tempol (1mM) for 1 hour before DHE staining. The level of O2− was determined using an inverted confocal microscope (Nikon TE3000).
Immunohistochemistry staining
SMA segments were fixed with 4% formaldehyde for 1 hour. Cryosections (7 μm) were blocked with 1% BSA and stained using rabbit polyclonal antibodies against SK3 (H-45, SC28621; Santa Cruz Biotechnology) or IK1 (H-120, SC32949; Santa Cruz Biotechnology), or a mouse monoclonal antibody against 3-nitrotyrosine (3-NT, SC32757; Santa Cruz Biotechnology 1:50), and costained with a purified rat antibody against mouse CD31, an endothelium marker (BD Biosciences; 1:100). FITC-conjugated anti–rabbit and anti–mouse, and Texas red-conjugated anti–rat secondary antibodies (1:200) were used to detect SK3, IK1, 3-NT, and CD31 expression, respectively. Negative controls using the corresponding IgG isotypes were included to check for nonspecific staining. Images were taken using an inverted confocal microscope (Nikon TE3000) and tissues were examined by investigators with no knowledge of the mouse group from which they were taken.
Cell culture
HCMECs (CC7030; Lonza) were cultured in Microvascular Endothelial Cell Growth Medium-2 (EGM-2MV; CC3202, Lonza) following the directions of the manufacturer. The cells were used between passages 6-8, cultured to 80%-90% confluence, and then treated with L-Hcy at the indicated concentrations for 48 hours in the presence or absence of selected pharmacologic inhibitors, including PEG-SOD (150 U/mL), PEG-Cat (150 U/mL), and uric acid (100μM).
Western blot and immunoprecipitation
Protein extracts were prepared from SMAs or cultured HCMECs. Proteins (40 μg) were fractionated by 9% SDS-PAGE and transferred to nitrocellulose blots. The expression of SK3 and IK1 was determined by Western blotting using rabbit polyclonal antibodies against SK3 (H-45, SC28621; Santa Cruz Biotechnology) and IK1 (H-120, SC32949; Santa Cruz Biotechnology). SK3 and IK1 nitration was determined by immunoprecipitation of 200 μg of protein with a mouse monoclonal antibody against 3-NT (SC32757), followed by blotting with antibodies against SK3 and IK1.
Chemicals
L-Hcy was freshly prepared by reducing L-homocystine with a 2-fold molar excess of DTT for 30 minutes at 37°C, pH 8.0, as described previously.20 If not otherwise specified, all chemicals were purchased from Sigma-Aldrich.
Statistics
In vitro studies were repeated at least 3 times with triplicates/group/experiment. Statistical analyses were performed with SigmaStat 2.03 (SPSS Science). Results are expressed as the means ± SEM. For statistical comparison of single parameters, independent t test was used for 2 groups and 1-way ANOVA with Bonferroni adjustment was performed for multiple groups. P < .05 was considered significant.
Results
Characterization of mice and Hcy measurement
Tg-hCBS Cbs−/− mice developed severe HHcy and had a plasma Hcy level of 169.5 ± 3.5μM at 8-10 months of age. Plasma total Hcy levels were 5.6 ± 1.2μM in the control mice (Figure 1A). Body weight and heart weight were not changed (not shown).
HHcy impaired endothelium-dependent and EDHF-mediated vascular relaxation in SMAs of Tg-hCBS Cbs mice. SMAs were isolated from Tg-hCBS Cbs mice. (A) Plasma total Hcy levels. (B-C) Vascular contractile responses to KCl (120mM) and to cumulative additions of PE. (D) Endothelium-independent relaxation. SMAs were precontracted with PE (1μM) and examined for relaxation to cumulative additions of SNP. (E) Endothelium-dependent relaxation. SMAs were precontracted with PE and examined for relaxation to ACH. (F) NO-independent relaxation. SMAs were pretreated with the NOS inhibitor L-NAME (100μM), precontracted with PE, and examined for relaxation to ACH. (G) PGI2-independent relaxation. SMAs were pretreated with the nonselective COX inhibitor INDO (10μM), precontracted with PE, and examined for relaxation to ACH. (H) PGI2-mediated relaxation. SMAs were pretreated with the NOS inhibitor L-NAME (100μM) and the nonselective KCa blocker TEA (1mM), precontracted with PE, and examined for relaxation to ACH. (I) NO-mediated endothelium-dependent relaxation. SMAs were pretreated with the nonselective COX inhibitor INDO (10μM) and the nonselective KCa blocker TEA, precontracted with PE, and examined for relaxation to ACH. (J) EDHF-mediated endothelium-dependent relaxation. SMAs were pretreated with INDO and the NOS inhibitor L-NAME, precontracted with PE, and examined for relaxation to ACH. Values are means ± SEM (n = 5-7 mice, 2 vessel segments/mouse). *P < .05 compared with control mice. CT indicates the control group.
HHcy impaired endothelium-dependent and EDHF-mediated vascular relaxation in SMAs of Tg-hCBS Cbs mice. SMAs were isolated from Tg-hCBS Cbs mice. (A) Plasma total Hcy levels. (B-C) Vascular contractile responses to KCl (120mM) and to cumulative additions of PE. (D) Endothelium-independent relaxation. SMAs were precontracted with PE (1μM) and examined for relaxation to cumulative additions of SNP. (E) Endothelium-dependent relaxation. SMAs were precontracted with PE and examined for relaxation to ACH. (F) NO-independent relaxation. SMAs were pretreated with the NOS inhibitor L-NAME (100μM), precontracted with PE, and examined for relaxation to ACH. (G) PGI2-independent relaxation. SMAs were pretreated with the nonselective COX inhibitor INDO (10μM), precontracted with PE, and examined for relaxation to ACH. (H) PGI2-mediated relaxation. SMAs were pretreated with the NOS inhibitor L-NAME (100μM) and the nonselective KCa blocker TEA (1mM), precontracted with PE, and examined for relaxation to ACH. (I) NO-mediated endothelium-dependent relaxation. SMAs were pretreated with the nonselective COX inhibitor INDO (10μM) and the nonselective KCa blocker TEA, precontracted with PE, and examined for relaxation to ACH. (J) EDHF-mediated endothelium-dependent relaxation. SMAs were pretreated with INDO and the NOS inhibitor L-NAME, precontracted with PE, and examined for relaxation to ACH. Values are means ± SEM (n = 5-7 mice, 2 vessel segments/mouse). *P < .05 compared with control mice. CT indicates the control group.
SMA vascular contractile response was not changed in severe HHcy
Vascular contractile responses to the single dose of KCl, a determination of depolarization-induced vessel contraction, and cumulative doses of PE, a measurement of α1-adrenoceptor–induced contraction, were similar in SMA segments from Tg-hCBS Cbs−/− mice and from control mice (Figure 1B-C).
HHcy impairs endothelium-dependent relaxation, but not endothelium-independent, relaxation in SMAs
ACH, an endothelium-dependent relaxant, produced a dose-dependent relaxation that was impaired in SMAs of Tg-hCBS Cbs−/− mice compared with control mice (Figure 1E). Endothelium-independent vascular relaxation to SNP, an NO donor used to determine the sensitivity of VSMCs to NO, was similar in SMAs obtained from control and hyperhomocysteinemic mice (Figure 1D). Endothelium-dependent vascular relaxation responses to ACH, as evaluated by maximum relaxation, were 98.0% ± 0.5% in control mice and reduced to 57.8% ± 6.5% in SMAs from Tg-hCBS Cbs−/− mice (Figure 1E). These data indicate that HHcy impairs endothelium-dependent, but not endothelium-independent, relaxation in SMAs.
HHcy impairs NO-mediated and EDHF-mediated endothelium-dependent relaxation in SMAs
Because endothelium-dependent relaxation is mediated by EDHF, NO, and PGI2, we examined the effect of HHcy on these 3 distinct relaxation pathways. NO-independent endothelium-dependent relaxation, determined by preincubating the SMAs with the NOS inhibitor L-NAME, was 83.8% in control mice and reduced to 50.7% in Tg-hCBS Cbs−/− mice (Figure 1F). PGI2-independent endothelium-dependent relaxation, determined by preincubating the SMAs with the nonselective COX inhibitor INDO, was 94.5% in control mice and reduced to 62.5% in Tg-hCBS Cbs−/− mice (Figure 1G). The maximal PGI2-mediated endothelium-dependent relaxation, determined by preincubating the SMAs with the NOS inhibitor L-NAME and the nonselective KCa blocker TEA, was 25.9% ± 6.4% in control mice (26.4% of the total endothelium-dependent relaxation), and this was not changed in Tg-hCBS Cbs−/− mice (26.9% ± 3.5%; Figure 1H). The maximal NO-mediated endothelium-dependent relaxation, determined by preincubating the SMAs with the nonselective COX inhibitor INDO and TEA, was 63.1% ± 10.4% in control mice (64.4% of the total endothelium-dependent relaxation) and this was reduced to 33.0% ± 7.8% in hCBS Cbs−/− mice (Figure 1I). The maximal EDHF-mediated relaxation, determined by preincubating the SMAs with L-NAME and INDO, was 86.3% ± 4.8% in control mice (88.1% of the total endothelium-dependent relaxation) and this was reduced to 40.4% ± 6.0% in Tg-hCBS Cbs−/− mice, a 2.1-fold reduction (Figure 1J). These data suggest that EDHF contributes to the majority of endothelium-dependent relaxation (88.1%) in SMAs, and that HHcy impairs endothelium-dependent relaxation in large part by inhibiting EDHF-mediated relaxation of SMAs.
HHcy impairs EDHF-mediated vascular relaxation via inhibition SKs and IKs in SMAs. EDHF-mediated endothelium-dependent relaxation is primarily controlled by opening of KCas.21 Endothelial KCa currents are mediated mainly by 2 KCa subtypes, IK and SK, but not BK.10 We found that TEA, a nonselective KCa blocker, completely blocked EDHF-mediated relaxation in SMAs (Figure 2A). The SK-specific blocker apamin reduced EDHF-mediated relaxation from 86.3% ± 4.8% (Figure 1I) to 58.2% ± 12.1% (Figure 2B) in control mice and from 40.4% ± 6.0% (Figure 1I) to 31.0% ± 2.9% (Figure 2B) in Tg-hCBS Cbs−/− mice. The IK blocker Tram34 reduced EDHF-mediated relaxation to 62.5% ± 7.5% in control mice and to 39.3% ± 3.4% in Tg-hCBS Cbs−/− mice (Figure 2C). Combined, the SK and IK blockers reduced EDHF-mediated relaxation to 17.0% ± 3.6% in control mice and to 29.4% ± 6.7% in Tg-hCBS Cbs−/− mice (Figure 2D). HHcy reduced KCa-controlled EDHF-mediated maximal relaxation from 100% in the control mice to 53.5% ± 3.8% in HHcy mice; SK-controlled relaxation from 32.6% ± 2.5% of KCa relaxation in control mice to 10.5% ± 4.9% in Tg-hCBS Cbs−/− mice (3.1-fold reduction); IK-controlled relaxation from 32.9% ± 10.2% to 1.3% ± 0.2% (25.3-fold reduction); and SK/IK-controlled relaxation from 65.9% ± 5.2% to 26.8% ± 5.4% (2.5-fold reduction; Figure 2E). These data indicate that HHcy impairs EDHF-mediated endothelium-dependent relaxation in SMAs primarily via IK inactivation along with SK inactivation.
HHcy-impaired EDHF-mediated vascular relaxation via inhibition of SKs and IKs in SMAs. SMAs were isolated from Tg-hCBS Cbs mice. (A) KCa blocker–inhibited EDHF-mediated relaxation. SMAs were pretreated with the COX inhibitor INDO (10μM), the NOS inhibitor L-NAME (100μM), and the KCa blocker TEA (1mM), precontracted with PE, and examined for relaxation to the cumulative additions of ACH. (B) SK blocker–inhibited EDHF-mediated relaxation. SMAs were pretreated with INDO, L-NAME, and the SK blocker apamin (1μM), precontracted with PE, and examined for relaxation to ACH. (C) IK blocker–inhibited EDHF-mediated relaxation. SMAs were pretreated with INDO, L-NAME, and the IK blocker Tram34 (1μM), precontracted with PE, and examined for relaxation to ACH. (D) SK/IK blocker–inhibited EDHF-mediated relaxation. SMAs were pretreated with INDO, L-NAME, apamin, and Tram34, precontracted with PE, and examined for relaxation to ACH. (E) Kca-controlled maximal relaxation of Kca. Maximal relaxation was calculated by subtracting the blocker inhibited EDHF-mediated relaxation (−5.5 log M; Figure 2A-C) from that of EDHF-mediated relaxation in the absence of blocker (Figure 1I). Value in the CT group treated with KCa blocker was defined as 100%. Values are means ± SEM (n = 5-7 mice, 2 vessel segments/mouse). *P < .05 compared with control mice. CT indicates the control group.
HHcy-impaired EDHF-mediated vascular relaxation via inhibition of SKs and IKs in SMAs. SMAs were isolated from Tg-hCBS Cbs mice. (A) KCa blocker–inhibited EDHF-mediated relaxation. SMAs were pretreated with the COX inhibitor INDO (10μM), the NOS inhibitor L-NAME (100μM), and the KCa blocker TEA (1mM), precontracted with PE, and examined for relaxation to the cumulative additions of ACH. (B) SK blocker–inhibited EDHF-mediated relaxation. SMAs were pretreated with INDO, L-NAME, and the SK blocker apamin (1μM), precontracted with PE, and examined for relaxation to ACH. (C) IK blocker–inhibited EDHF-mediated relaxation. SMAs were pretreated with INDO, L-NAME, and the IK blocker Tram34 (1μM), precontracted with PE, and examined for relaxation to ACH. (D) SK/IK blocker–inhibited EDHF-mediated relaxation. SMAs were pretreated with INDO, L-NAME, apamin, and Tram34, precontracted with PE, and examined for relaxation to ACH. (E) Kca-controlled maximal relaxation of Kca. Maximal relaxation was calculated by subtracting the blocker inhibited EDHF-mediated relaxation (−5.5 log M; Figure 2A-C) from that of EDHF-mediated relaxation in the absence of blocker (Figure 1I). Value in the CT group treated with KCa blocker was defined as 100%. Values are means ± SEM (n = 5-7 mice, 2 vessel segments/mouse). *P < .05 compared with control mice. CT indicates the control group.
HHcy stimulates SK3 and IK1 protein expression in the endothelium of SMAs
To identify the regulatory mechanism of HHcy-induced KCa inactivation, we examined the protein levels of IKs and SKs in SMAs. SK3 and IK1 were detected in the endothelium and colocalized with endothelial marker CD31 in SMAs from both mice (Figure 3A-B). The expressions of SK3 and IK1 protein were increased in SMAs from Tg-hCBS Cbs−/− mice by immunohistochemical staining (Figure 3A-B). SK3 and IK1 proteins were increased by 3.3- and 3.6-fold, respectively, in Tg-hCBS Cbs−/− mice by Western blot analysis of SMAs protein extracts (Figure 3C-D). Therefore, HHcy increased SK3 and IK1 protein expression in the endothelium of SMAs.
HHcy-stimulated SK and IK expression in SMAs of Tg-hCBS Cbs mice. Mouse SMAs cross-sections were immunostained with antibodies against SK3 (green, A) or IK1 (green, B), and costained with the endothelial marker CD31 (red). Merged image (yellow to orange) shows that SK3 and IK1 are localized in the endothelium and increased in Cbs−/− mice. (C-D) Immunoblot of SK3 and IK1 in SMA extracts and quantification. Values are means ± SEM (n = 5-7 mice). *P < .05 compared with control mice. CT indicates the control group.
HHcy-stimulated SK and IK expression in SMAs of Tg-hCBS Cbs mice. Mouse SMAs cross-sections were immunostained with antibodies against SK3 (green, A) or IK1 (green, B), and costained with the endothelial marker CD31 (red). Merged image (yellow to orange) shows that SK3 and IK1 are localized in the endothelium and increased in Cbs−/− mice. (C-D) Immunoblot of SK3 and IK1 in SMA extracts and quantification. Values are means ± SEM (n = 5-7 mice). *P < .05 compared with control mice. CT indicates the control group.
HHcy induces oxidative stress in SMAs of Tg-hCBS Cbs mice
One potential mechanism leading to impaired KCa function is oxidative stress.22 We examined vessel wall O2− production and found that severe HHcy increased O2− production by 1.78-fold, as measured by DHE staining of SMAs both by longitudinal view and cross-section analysis (Figure 4A-B). Preincubation of SMAs with the antioxidants PEG-SOD or Tempol significantly reduced O2− generation in SMAs of control and Tg-hCBS Cbs−/− mice (Figure 4A-B). Because ONOO− is a strong and relatively stable oxidant species capable of causing nitrosylation of tyrosine residues on key cellular proteins, we measured the content of 3-NT, a marker of ONOO− production, in SMA cross-sections by immunohistochemical staining. We found that HHcy increased 3-NT staining in the endothelium of SMAs (Figure 4C). These data indicate that HHcy induces oxidative stress in SMAs at least in part via the formation of ONOO−.
HHcy-induced oxidative stress in SMAs of Tg-hCBS Cbs mice. Mouse SMA segments and cross-sections were incubated with the antioxidants PEG-SOD (150 U/mL) or the O2− scavenger Tempol (1mM), and stained with the O2− marker DHE (red, A) or 3-NT (green, C), and costained with endothelial marker CD31 (red, C) antibodies. Merged image (yellow to orange, C) shows that 3-NT is localized to the endothelium and increased in Cbs−/− mice. (B) Quantitative analysis of O2− generation by DHE staining in panel A. Values are means ± SEM (n = 5-7 mice). *P < .05 compared with vehicle in control mice; †P < .05 compared with vehicle in Cbs−/− mice. CT indicates the control group.
HHcy-induced oxidative stress in SMAs of Tg-hCBS Cbs mice. Mouse SMA segments and cross-sections were incubated with the antioxidants PEG-SOD (150 U/mL) or the O2− scavenger Tempol (1mM), and stained with the O2− marker DHE (red, A) or 3-NT (green, C), and costained with endothelial marker CD31 (red, C) antibodies. Merged image (yellow to orange, C) shows that 3-NT is localized to the endothelium and increased in Cbs−/− mice. (B) Quantitative analysis of O2− generation by DHE staining in panel A. Values are means ± SEM (n = 5-7 mice). *P < .05 compared with vehicle in control mice; †P < .05 compared with vehicle in Cbs−/− mice. CT indicates the control group.
HHcy impairs endothelium-dependent and EDHF-mediated relaxation via oxidative stress in SMAs
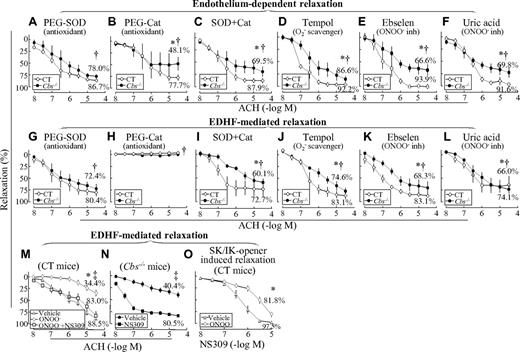
To assess the potential biochemical mechanism underlying HHcy-impaired endothelium-dependent and EDHF-mediated relaxation in SMAs, vessel segments were preincubated with various pharmacologic inhibitors for 1 hour before assessing the relaxation response to ACH. SOD, a O2− anion scavenger, improved endothelium-dependent and EDHF-mediated relaxation from 57.8% and 40.4% (Figure 1E and J) to 78.0% and 72.4% (Figure 5A and G) in SMAs of Tg-hCBS Cbs−/− mice, respectively. Catalase, a hydrogen peroxide–metabolizing enzyme, reduced endothelium-dependent relaxation from 98.0% and 57.8% (Figure 1E) to 77.7% and 48.1% (Figure 5B) in SMAs of control and Tg-hCBS Cbs−/− mice, and completely prevented EDHF-mediated relaxation in SMAs from all mice, respectively (Figure 5H). The combination of SOD and catalase improved endothelium-dependent and EDHF-mediated relaxation from 57.8% and 40.4% (Figure 1E and J) to 69.5% and 60.1% (Figure 5C and I) in SMAs of Tg-hCBS Cbs−/− mice, respectively. Tempol, a O2− anion scavenger, improved endothelium-dependent and EDHF-mediated relaxation to 86.6% and 74.6% (Figure 5D and J), respectively. The ONOO− inhibitors ebselen and uric acid improved endothelium-dependent relaxation to 66.6% ± 9.3% and 69.8% ± 9.5%, and EDHF-mediated relaxation to 68.3% ± 11.6% and 66.0% ± 16.5%, respectively (Figure 5E-F and K-L). We also found that ONOO− reduced EDHF-mediated relaxation in SMAs of control mice from 88.5% to 34.4%, which was rescued by the SK/IK opener NS309 to 83.0% (Figure 5M), and that NS309 restored EDHF-mediated relaxation in SMAs of Tg-hCBS Cbs−/− mice from 40.4% to 80.5% (Figure 5N). Finally, SK/IK-controlled relaxation (induced by the SK/IK opener NS309) was inhibited by ONOO− from 97.5% ± 0.7% to 81.8% ± 4.1%, respectively, in SMAs of control mice (Figure 5O).
HHcy-impaired endothelium-dependent and EDHF-mediated relaxation occurs via oxidative stress in SMAs of Tg-hCBS Cbs mice. (A-F) Endothelium-dependent relaxation response to cumulative concentrations of ACH in SMAs pretreated with indicated inhibitors for 1 hour. (G-L) EDHF-mediated relaxation to cumulative additions of ACH in SMAs pretreated with indicated inhibitors for 1 hour, and with the COX inhibitor INDO (1μM) plus the NOS inhibitor L-NAME (100μM) for another 30 minutes. (M) ONOO−-inhibited EDHF-mediated relaxation in SMAs of control mice. EDHF-mediated relaxation to cumulative concentrations of ACH was assessed in SMAs pretreated without and with ONOO− (100μM) for 30 minutes, followed by the SK/IK opener NS309 (1μM) in the presence of INDO and L-NAME for an additional 30 minutes. (N) The SK/IK opener NS309 restored EDHF-mediated relaxation in Cbs−/− mice. EDHF-mediated relaxation to cumulative concentrations of ACH was assessed in SMAs pretreated with the SK/IK opener NS309 (10μM) in the presence of INDO and L-NAME for 30 minutes. (O) ONOO−-inhibited SK/IK opener-mediated relaxation in SMAs of control mice. Vascular relaxation response to cumulative additions of NS309 (10nM-10μM) was assessed in SMAs pretreated without and with ONOO− (100μM) for 30 minutes followed by INDO and L-NAME for an additional 30 minutes. SMAs were precontracted with PE (1μM). Values are means ± SEM (n = 5-7 mice). *P < .05 compared with control mice in the experiment shown in Figure 1D and G; †P < .05 compared with CBS−/− mice in Figure 1D and G; and ‡P < .05 compared with the NS309-treated group in Figure 1D and G. CT indicates the control group.
HHcy-impaired endothelium-dependent and EDHF-mediated relaxation occurs via oxidative stress in SMAs of Tg-hCBS Cbs mice. (A-F) Endothelium-dependent relaxation response to cumulative concentrations of ACH in SMAs pretreated with indicated inhibitors for 1 hour. (G-L) EDHF-mediated relaxation to cumulative additions of ACH in SMAs pretreated with indicated inhibitors for 1 hour, and with the COX inhibitor INDO (1μM) plus the NOS inhibitor L-NAME (100μM) for another 30 minutes. (M) ONOO−-inhibited EDHF-mediated relaxation in SMAs of control mice. EDHF-mediated relaxation to cumulative concentrations of ACH was assessed in SMAs pretreated without and with ONOO− (100μM) for 30 minutes, followed by the SK/IK opener NS309 (1μM) in the presence of INDO and L-NAME for an additional 30 minutes. (N) The SK/IK opener NS309 restored EDHF-mediated relaxation in Cbs−/− mice. EDHF-mediated relaxation to cumulative concentrations of ACH was assessed in SMAs pretreated with the SK/IK opener NS309 (10μM) in the presence of INDO and L-NAME for 30 minutes. (O) ONOO−-inhibited SK/IK opener-mediated relaxation in SMAs of control mice. Vascular relaxation response to cumulative additions of NS309 (10nM-10μM) was assessed in SMAs pretreated without and with ONOO− (100μM) for 30 minutes followed by INDO and L-NAME for an additional 30 minutes. SMAs were precontracted with PE (1μM). Values are means ± SEM (n = 5-7 mice). *P < .05 compared with control mice in the experiment shown in Figure 1D and G; †P < .05 compared with CBS−/− mice in Figure 1D and G; and ‡P < .05 compared with the NS309-treated group in Figure 1D and G. CT indicates the control group.
L-Hcy increases O2− production in cultured HCMECs
HCMECs were chosen as a cardiovascularly relevant cellular model with which to study endothelium regulatory mechanisms in the microvasculature. We first examined the direct effect of Hcy on O2− production in HCMECs by DHE staining and found that L-Hcy increased red fluorescence in a dose-sensitive manner (Figure 6A). Hcy at the concentration of 500μM increased O2− production to 224% of that in the control cells (Figure 6C). This was reversed by antioxidants (SOD and Tempol, Figure 6B-D).
L-Hcy–increased O2− production in HCMECs. (A) Dose response. HCMECs were treated with L-Hcy at the indicated concentration for 48 hours and then treated with the indicated inhibitors for 60 minutes before the addition of DHE (3μM) for 30 minutes, including PEG-SOD (150 U/mL) and Tempol (1mM). (A-B) Photomicrographs of DHE-stained HCMECs. Images were acquired by 2-laser inverted confocal microscopy (Nikon TE3000). (C-D) Quantitative analysis of O2− production by DHE staining in panels A and B. Means ± SEM values from at least 3 separate experiments are shown. *P < .05 compared with untreated cells or vehicle in control mice. †P < .05 compared with vehicle in L-Hcy (500μM). HCMECs treated with angiotensin II (10μM) were used as a positive control. CT indicates the control group.
L-Hcy–increased O2− production in HCMECs. (A) Dose response. HCMECs were treated with L-Hcy at the indicated concentration for 48 hours and then treated with the indicated inhibitors for 60 minutes before the addition of DHE (3μM) for 30 minutes, including PEG-SOD (150 U/mL) and Tempol (1mM). (A-B) Photomicrographs of DHE-stained HCMECs. Images were acquired by 2-laser inverted confocal microscopy (Nikon TE3000). (C-D) Quantitative analysis of O2− production by DHE staining in panels A and B. Means ± SEM values from at least 3 separate experiments are shown. *P < .05 compared with untreated cells or vehicle in control mice. †P < .05 compared with vehicle in L-Hcy (500μM). HCMECs treated with angiotensin II (10μM) were used as a positive control. CT indicates the control group.
L-Hcy increased IK1 expression and tyrosine nitration of SKs and IKs in HCMECs
We found that L-Hcy (200 and 500μM) increased IK1 protein expression by 141% and 195%, respectively, but did not change SK3 protein levels in HCMECs (Figure 7A-C). Interestingly, L-Hcy (500μM) dramatically increased tyrosine nitration of SKs and IKs by 374% and 435%, respectively. The antioxidants SOD, catalase, and uric acid reduced SK3 tyrosine nitration to 157%, 180%, and 157% and IK1 tyrosine nitration to 199%, 271%, and 73% in Hcy (500μM)–treated cells (Figure 7D-F).
L-Hcy–increased IK1 expression and oxidation/nitration of SKs and IKs in HCMECs. HCMECs were treated with or without L-Hcy at the indicated concentrations for 48 hours. Western blot analysis was performed for SK3 and IK1 in HCMECs extracts before (A) and after (D) immunoprecipitation with 3-NT antibody. Values are means ± SEM (n = 3). *P < .05 compared with no-treatment parallel control; †P < .05 compared with Hcy 200μM; and #P < .05 compared with Hcy 500μM. CT indicates the control group; UA, uric acid.
L-Hcy–increased IK1 expression and oxidation/nitration of SKs and IKs in HCMECs. HCMECs were treated with or without L-Hcy at the indicated concentrations for 48 hours. Western blot analysis was performed for SK3 and IK1 in HCMECs extracts before (A) and after (D) immunoprecipitation with 3-NT antibody. Values are means ± SEM (n = 3). *P < .05 compared with no-treatment parallel control; †P < .05 compared with Hcy 200μM; and #P < .05 compared with Hcy 500μM. CT indicates the control group; UA, uric acid.
Discussion
In the present study, we determined that HHcy induced ED. We report 2 major novel findings. First, HHcy-impaired endothelium-dependent relaxation in SMAs occurs predominantly via EDHF inhibition. Second, HHcy impaired EDHF-mediated relaxation by inactivating SKs and IKs via oxidative and nitrosative stress.
HHcy-induced ED has been reported in both human studies5,6,23 and animal models of HHcy.1-4,24 However, the fundamental mechanisms by which HHcy induces ED remain unclear. Loss of NO bioavailability secondary to an increase in oxidative stress has been suggested to be a leading mechanism in conduit vessels such as the aorta, in which NO is the predominant endothelium-derived vasodilator. However, very little is known about how HHcy affects microvasculature function, which is dominantly controlled by an EDHF-related mechanism. The contribution of EDHF is small or even negligible in relation to the total vasodilatory response in large conduit arteries, but it accounts for almost half of the response in small arteries (diameter < 500 μm), such as mesenteric and carotid arteries, and predominates in resistance arterioles (diameter < 100 μm), such as SMAs and middle cerebral arterioles.8
We found that the EDHF pathway was dominant and responsible for 88% of endothelial-dependent relaxation in SMAs of control mice, whereas the NO and PGI2 pathways accounted for 63.1% and 26.9% of the relaxation response, respectively. We observed that SOD and catalase reduced endothelial-dependent relaxation from 98% to 86.7% and 77.7%, respectively, in the control mice. More interestingly, catalase completely blocked EDHF-dependent relaxation in the SMAs of control mice. Our data support the notions that SMAs rely primarily on EDHF and secondarily on NO for agonist-induced endothelium-dependent dilation,25,26 and that H2O2 mediates EDHF-dependent dilation.27 We also provide evidence supporting the hypothesis that basal levels of O2− are needed for endothelial-dependent relaxation under physiologic conditions.28 Further, we reveal some degree of compensatory overlap between the 3 regulatory pathways (EDHF, NO, and PGI2) in controlling endothelium-dependent dilation in small-resistance arterioles.
Using our newly developed mouse model of HHcy (plasma Hcy ∼170μM), we found that severe HHcy reduces endothelium-dependent NO- and EDHF-mediated vascular relaxation to ACH from 63.1% to 33.0% and from 86.3% to 40.4%, respectively. The effect of HHcy on EDHF regulation has not been previously evaluated in resistance arterioles or in animal models of severe HHcy. Our data are in accordance with the attenuation of EDHF-mediated responses noted in rat renal arteries (diameter > 500 μm) of HHcy rats (plasma Hcy 33-74μM).3,11 Because the microvasculature performs an essential role in regulating tissue perfusion, our findings suggest that severe HHcy may contribute to tissue ischemia primarily via EDHF inhibition.
It has been suggested that EDHF-mediated responses are initiated by increases in intracellular calcium within the endothelium, which activates SKs and IKs, causing K+ efflux and membrane hyperpolarization.10 It is now understood that endothelial SK3 and IK1 channels have distinct stimulus-dependent functions and together constitute the major electric trigger for EDHF dilator responses.2,10 This is supported by the disrupted EDHF function observed in SK3- and IK1-deficient mice14 and by their endothelial location. SK3 is predominantly located at interendothelial junctions, whereas IK1 is localized to the endothelial-smooth muscle cell interface at internal elastic lamina holes.29 Vascular relaxation to NS309, the SK/IK opener, is impaired in precontracted arterioles (80-180 μm in diameter) from patients in cardioplegic arrest.30 Furthermore, studies in human coronary and skeletal muscle arterioles using NS309 suggest that the human vascular bed responds very well to drugs that target SK and IK channels.30,31 We found that a nonselective KCa blocker completely diminished EDHF-mediated relaxation. A similar finding was obtained with a combination of SK and IK blockers. In control animals, SK and IK each contribute approximately 32% of KCa-controlled EDHF-mediated relaxation. HHcy abolishes SK-mediated EDHF relaxation, which is reduced from 32.6% in control animals to 10.5% in hyperhomocysteinemic mice, and HHcy largely abolishes IK-mediated EDHF relaxation from 32.9% to 1.3%. Our study is the first to identify a role of KCa inhibition in the impairment of EDHF-mediated relaxation in HHcy and to characterize the potent blockade of IK activity and the less potent inhibition of SK activity in this setting. This is in good accordance with the notion that IK is a determinant in EDHF-mediated relaxation in the resistant artery.32
It has been suggested that KCa activity can be modulated by gene expression14 and protein oxidation/nitrosation.33,34 The KCa channels are tetrameric, with each subunit possessing 6-7 transmembrane domains. Knowledge from crystallization and structure analyses has indicated that K+ channels share common structural topology. The 4 identical monomers are connected by a P-loop region to form a central pore that is regulated by conformational changes to allow reversibly opening/occluding the pore through which K+ ions permeate.35 A tyrosine-containing GYG motif is the “signature sequence” in the P-loop region determining the selective K+ entrance.36 Recent molecular modeling has indicated that the IK P-loop region contains 3 tyrosine residues and 1 cysteine residue.37 The tyrosine and cysteine residues in the P-loop region and in the GYG motif of SK and IK protein are potential targets of ONOO− for tyrosine nitration and cysteine S-nitrosylation for conformational regulation of the channels.
We found that HHcy-impaired IK- and SK-mediated EDHF relaxation is associated with increased protein levels of SK and IK and elevated O2− production in SMAs and in cultured HCMECs, with increased 3-NT levels in SMAs and increased SK/IK tyrosine nitration in cultured HCMECs. Furthermore, we found that ONOO− inhibited EDHF-mediated and SK/IK-opener NS309–induced relaxation. Finally, the SK/IK-opener NS309 restored HHcy- and ONOO−-induced impairment in EDHF-mediated relaxation. We postulate that SK/IK tyrosine nitration results in inactivation of the channels in HHcy. In response to the channel inactivation, the amount of IK and SK protein increases. However, the compensation is not effective due to tyrosine nitration.
Our data strongly support the recent finding that SK and IK expression does not necessarily correlate with the functional significance of these channels,38 and lead us to hypothesize that HHcy inhibits IK and SK function via an oxidative stress- and/or nitration-related mechanism. This hypothesis is supported by our data showing that the antioxidant SOD and the ONOO− scavenger uric acid largely correct both HHcy-induced impairment in endothelium-dependent relaxation and EDHF-mediated relaxation in SMAs and O2− production in SMAs and HCMECs. In addition, SOD, catalase, and uric acid corrected Hcy-induced SK/IK protein tyrosine nitration in HCMECs.
Our data support a new hypothesis that Hcy increases O2− production, leading to increased ONOO− formation (the reaction of NO and O2− is strong and relatively stable). This results in decreased NO bioavailability and increased IK1 and SK3 tyrosine nitration, leading to IK1/SK3 inactivation and impairment of EDHF-mediated relaxation. We conclude that HHcy impairs EDHF-mediated vascular relaxation in microvessels such as SMAs via SK and IK inactivation through oxidative and nitrosative stress. Therefore, SK/IK may be considered novel targets for the prevention and treatment of HHcy-mediated endothelial dysfunction in resistance arterioles.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health grants HL67033, HL77288, and HL82774 (H.W.); HL74966 (W.D.); HL94451 (X.F.Y.); HL86699 and SLORR27327 (M.M); and HL57299 (W.D.K.).
National Institutes of Health
Authorship
Contribution: Z.C. performed the research and wrote a draft of the manuscript; X.J., S.G., and K.M. performed the research; W.D.K., D.P., and M.M. helped with the experimental design and analyzed the data; A.I.S., W.D., and X.Y. helped with the experimental design, analyzed the data, and wrote the manuscript; and H.W. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hong Wang, Department of Pharmacology, Temple University School of Medicine, 3420 North Broad St, Philadelphia, PA 19140; e-mail: hongw@temple.edu.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal