Abstract
Foxp3+ regulatory T cells play a pivotal role in maintaining self-tolerance and immune homeostasis. In the absence of regulatory T cells, generalized immune activation and multiorgan T cell–driven pathology occurs. Although the phenomenon of immunologic control by Foxp3+ regulatory T cells is well recognized, the comparative effect over different arms of the immune system has not been thoroughly investigated. Here, we generated a cohort of mice with a continuum of regulatory T-cell frequencies ranging from physiologic levels to complete deficiency. This titration of regulatory T-cell depletion was used to determine how different effector subsets are controlled. We found that in vivo Foxp3+ regulatory T-cell frequency had a proportionate relationship with generalized T-cell activation and Th1 magnitude, but it had a surprising disproportionate relationship with Th2 magnitude. The asymmetric regulation was associated with efficient suppression of Th2 cells through additional regulations on the apoptosis rate in Th2 cells and not Th1 cells and could be replicated by CTLA4-Ig or anti–IL-2 Ab. These results indicate that the Th2 arm of the immune system is under tighter control by regulatory T cells than the Th1 arm, suggesting that Th2-driven diseases may be more responsive to regulatory T-cell manipulation.
Introduction
Foxp3+ Regulatory T cells (Tregs) are a key modulator of immune system activation, with the ability to suppress the proliferation and cytokine production of CD4+ and CD8+ T cells.1-3 Although Tregs require Ag-specific stimulation for activation, after activation the suppressive function acquired is Ag nonspecific.4 This allows Tregs to act as a nonspecific rheostat on immune activation, decreasing the rate of spontaneous effector T-cell activation and thereby increasing dependence on pathogen-associated danger stimuli.5 As such, Tregs not only prevent autoimmunity because of autoreactive T cells6 but also reduce the activity of beneficial antipathogen7 and antitumor8 responses.
The model of Tregs as indiscriminate suppressors is being challenged by data showing surprisingly sophisticated molecular underpinning of Treg suppression. Foxp3+ Tregs use different molecular strategies to suppress T cells in different anatomical locations and to control different effector subpopulations.9-14 On the receiving end of immune tolerance, there is a growing body of evidence that Th1 and Th2 cells have qualitative differences in sensitivity to intrinsic regulation. For example, Th1 cells have enhanced, and more rapid, activation-induced cell death (AICD).15 In Th1 cells, AICD is mediated by Fas-FasL signaling and regulated by CD44,16,17 whereas in Th2 cells AICD is mediated by granzyme B activity and regulated by VIP.18,19 Likewise, Th1 cells are sensitive to endogenous galectin 1-induced cell death, whereas Th2 cells are resistant because of differential sialylation.20 Because Treg cells have been reported to use both granzyme B and galectin 1 as regulatory mediators,21 these intrinsic differences in Th1 and Th2 cells have the potential to modulate basal suppression levels.
The possibility of asymmetric aggregate effects of Treg suppression on Th1 and Th2 populations is raised by the unusual and distinct characteristics of immunodysregulation polyendocrinopathy enteropathy X-linked syndrome. In these persons, with mutations in FOXP3,22 Th2 cytokine production is exaggerated,22 and severe autoimmunity is frequently coupled with allergic inflammation, atopy, eczema, food allergy, eosinophilic inflammation, and elevated IgE.23 Likewise, in mice deficient in Foxp3+ T cells the most substantial increases in immunoglobulin production are in the Th2-associated isotypes of IgG1 and IgE and the greatest increase in effector T-cell cytokine production occurs with IL-4.24 Partial T-cell immunodeficiency diseases also frequently manifest with Th2 dysregulation and are associated with restrictions in Treg number, function, or repertoire.25 These characteristics are notable because of the rarity of Th2 effector cells in the healthy context, despite the high availability of Th2-inducing cytokines.26
Here, we investigate the possibility of an asymmetric effect of Treg suppression by the generation of a quantitative titration experiment in Treg-depleted mice. We demonstrate that Tregs do indeed have differential aggregate effects over effector T-cell subsets, and it is this asymmetry that quenches an otherwise robust Th2 response in the healthy context. Furthermore, we find that the asymmetry is associated with apoptosis induction of the Th2, and not the Th1, response by Tregs and can be replicated in the absence of Tregs through substitution with CTLA-4 or neutralization of IL-2.
Methods
Mice
Foxp3DTR mice on the C57BL/6 background6 were analyzed at 8-12 weeks of age. Doses of 0, 2.5, 5.0, 7.5, 10, or 20 μg/kg diphtheria toxin (DT; Sigma-Aldrich) were administrated intraperitoneally to Foxp3DTR mice on days 0, 1, 3, and 6. BrdU (1 mg; Becton Dickinson) was administered daily by the intraperitoneal route, from days 0 to 8. CTLA4-Ig (Abatacept; Orencia) was delivered by intraperitoneal injection at 25 mg/kg on day 5. CD4+ T cells from Foxp3GFP mice27 were purified by the mouse CD4+ T-cell enrichment kit (Stemcell) according to the manufacturer's instructions and were adoptively transferred at 5 × 106 cells/mouse on day 7. S4B6 anti–IL-2 Ab was injected intraperitoneally at 50 μg/mouse per day from days 0 to 8. On day 9, all mice were killed, and spleen and lymph nodes were collected for analysis by ex vivo stimulation and intracellular staining. All animal procedures were approved by the University of Leuven Animal Ethics Committee.
Flow cytometry
Lymphocytes from pooled lymph nodes (cervical, mesenteric, axillary, brachial) or spleen were surface stained for 20 minutes at 4°C with anti–CD4-PE or PE-Cy5 (GK1.5; eBioscience), anti–CD44-PE-Cy5 (IM7; eBioscience), and anti–B220-PE-Cy7 (RA3-6B2; eBioscience) before fixation and permeabilization with the eBioscience Foxp3 staining kit. Cells were then stained for 20 minutes at 4°C with anti–GFP-Alexa Fluor 488 (Invitrogen) and anti–Foxp3-APC (FJK-16s; eBioscience).
Ex vivo stimulation and cytokine staining
Lymphocytes were plated at 5 × 105 cells/well in 96-well tissue culture plates (for Th1/Th2 staining) or at 4 × 106 cells/well in 24-well tissue culture plates (for Ki67/Caspase staining) in complete RPMI containing phorbol myristate acetate (50 ng/mL; Sigma-Aldrich), ionomycin (500 ng/mL; Sigma-Aldrich), and GolgiStop (1/1000; Becton Dickinson) and stimulated for 4-5 hours at 37°C. For activated Caspase 3 staining, FITC-DEVD-FMK (Abcam) was added in the last hour of stimulation. After stimulation, cells were surface stained and permeabilized as above, before intracellular staining with anti–IFNγ-APC or PE-Cy7 (XMG1.2; eBioscience), anti–IL-17A-APC (eBio17B7; eBioscience), anti–IL-4-PE-Cy7 (BVD6-24G2; eBioscience), anti–IL-10-APC (JES5-16E3; eBioscience), and anti–Ki-67-PE (B56; Becton Dickinson). BrdU staining required stimulated cells to be surface stained, fixed, and permeabilized with BrdU Flow Kits (Becton Dickinson) and frozen for 12 hours at −80°C in 10% dimethyl sulfoxide 90% FBS. Cells were then thawed, refixed, and treated with DNAse at room temperature for 30 minutes and stained as above for intracellular markers and anti–BrdU-APC.
Statistical analysis
Direct comparisons of 2 groups were performed by unpaired Student t tests, after an ANOVA analysis. The correlation between the percentage of Foxp3+ cells and cytokine responses was analyzed with JMP 7.0 software (SAS Institute). For each variable the relationship between the variable and the percentage of Foxp3+ cells was tested by fitting to linear, exponential, quadratic, and log models. The optimal model was selected with the least-squares approach. Further details for statistical analysis of relationships between the variables are presented in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Induced activation of T cells proportional to Treg number
To investigate the relationship between Treg number and effector T-cell activation and Th1 versus Th2 magnitude, we devised a system to selectively introduce acute quantitative decreases in the Foxp3+ Treg frequency. We used a knock-in mouse model in which a human DT receptor (DTR)–GFP (green fluorescent protein) fusion protein has been targeted into the Foxp3 locus (Foxp3DTR mice).6 In this system, Foxp3-expressing cells can be traced by GFP expression and are selectively depleted by the injection of DT. The DT-DTR system was conducive to this purpose, because toxicity at a level of 1 molecule per cell28 allows a limiting concentration of DT to eliminate a proportion of the Treg population while leaving the remainder intact. Furthermore, in contrast to partial Ab-mediated depletion strategies that can result in the selective depletion of particular subsets, DT exerts identical toxicity across all subpopulations.29
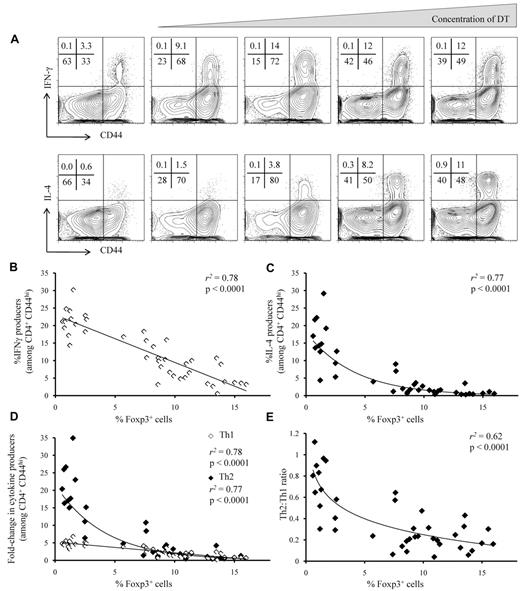
By administering a range of DT doses between 2.5 μg/kg and 20 μg/kg and taking advantage of stochastic variation in depletion efficiency, we generated a cohort of mice with a spectrum of Treg frequencies in the lymph nodes (Figure 1A-B). Treatment with DT on days 0, 1, 3, and 6 proved to be conducive to the generation of a stable intermediate Treg number out to day 9. Frequency distribution was relatively even, with the exception of an unstable range of Treg frequency at ∼ 3%-7% of CD4+ T cells. The bimodal distribution of Treg frequencies in the 10-μg/kg dose (> 7% or < 3%) may suggest that this range is unstable and that depletions in the 3%-7% range either show recovery to > 7% or collapse to < 3%. Notably, surviving Tregs at each point in the spectrum continue to express normal levels of both Foxp3 and the DTR-GFP fusion protein (Figure 1B), consistent with the expectation that the administration of intermediate doses of DT introduces quantitative reductions but not qualitative changes into the Treg population. Levels of depletion in the lymph nodes and spleens showed a high concordance rate (supplemental Figure 1A-B). The depletion of Tregs in adult mice results in a strong autoreactive immune response within 9 days of the initial DT treatment.6 To investigate general T-cell activation we measured CD44 expression on CD4+ T cells. In the lymph nodes the proportion of CD44hi CD4+ T cells showed an inverse correlation with the Treg frequency (Figure 1C; supplemental Methods). A similar relationship was observed in the spleen (Methods Figure 1C). Together, these results show the capacity of the Treg titration system to induce decrements in Treg frequency that lead to proportionate changes in the level of spontaneous T-cell activation in vivo.
Induction of T-cell activation proportional to titrated Treg depletion. Injection of limiting concentrations of DT resulted in a titration of Treg ablation in the lymph nodes of Foxp3DTR mice. (A) Representative flow cytometric profiles of a titration series of Foxp3DTR mice injected with limiting DT, displaying the percentage of CD4+ T cells positive for both Foxp3 and the DTR-GFP fusion protein. (B) Proportion of Foxp3+GFP+ Tregs among CD4+ T cells, 9 days after treatment with 0, 2.5, 5.0, 7.5, 10, and 20 μg/kg DT (n = 4, 5, 9, 5, 8, 8). Diamonds represent individual mice, bars represent condition average. (C) The relationship between Treg numbers (within CD4+ T cells) and expression of CD44 on CD4+Foxp3− T cells. Diamonds represent individual mice, and the trend line represents the optimal model for relationship fitting with the use of the least-squares approach.
Induction of T-cell activation proportional to titrated Treg depletion. Injection of limiting concentrations of DT resulted in a titration of Treg ablation in the lymph nodes of Foxp3DTR mice. (A) Representative flow cytometric profiles of a titration series of Foxp3DTR mice injected with limiting DT, displaying the percentage of CD4+ T cells positive for both Foxp3 and the DTR-GFP fusion protein. (B) Proportion of Foxp3+GFP+ Tregs among CD4+ T cells, 9 days after treatment with 0, 2.5, 5.0, 7.5, 10, and 20 μg/kg DT (n = 4, 5, 9, 5, 8, 8). Diamonds represent individual mice, bars represent condition average. (C) The relationship between Treg numbers (within CD4+ T cells) and expression of CD44 on CD4+Foxp3− T cells. Diamonds represent individual mice, and the trend line represents the optimal model for relationship fitting with the use of the least-squares approach.
Acute decrease in Treg frequency shows asymmetric control over Th2 effector T cells
The establishment of a titration curve of Treg depletion allowed the measurement of effector T-cell subset response to acute loss of Treg control. Th1-, Th2-, Th17-, and IL-10–producing effector subsets were defined by the expression of IFNγ, IL-4, IL-17, and IL-10, respectively. The null hypothesis was that the overall frequencies of these subsets would increase with increasing Treg depletion (in line with the increases observed in CD44+ T cells) but that their relative contributions would not change.
As was observed with CD44 expression, the frequency of in vivo–formed IFNγ-producing Th1 effector T cells increased proportionately with decreasing Treg frequency (Figure 2A-B; supplemental Methods). The relation between Treg frequency and the magnitude of the Th2 effector T-cell response, by contrast, was markedly disproportionate, with minimal increases in Th2-cell frequency under mild decreases in Treg frequency, but large-scale expansion of the Th2 response at lower Treg frequencies (Figure 2A,C; supplemental Methods). Relative to mice with normal Treg frequencies, mice completely depleted of Tregs showed a 5-fold increase in Th1 cells versus a 20-fold increase in Th2 cells, with qualitative differences in the Th1 versus Th2 accumulation response over intermediate Treg frequencies (Figure 2D; supplemental Methods). The difference in magnitude is because of reduced frequency of Th2 cells in mice with normal Treg frequencies, whereby the typical Th2/Th1 ratio is 1:5, compared with mice completely depleted of Tregs, whereby numerical parity is approached (Figure 2E). There was no evidence of plateau effects, because at no point did the rate of increase in Th1 or Th2 frequency begin to decline with further Treg depletion. Unlike Th1 and Th2 responses, the frequency of Th17- and IL-10–producing cells were relatively insensitive to Treg loss (supplemental Figure 2). Cytokine production in the lymph nodes was highly correlated with cytokine production in the spleen (supplemental Figure 3), which also showed a skewing toward Th2 bias as Treg frequency was reduced (supplemental Figure 4).
Asymmetric accumulation of Th1 and Th2 cells after titrated Treg depletion. Effector CD4+ T cells from the lymph nodes of DT-treated Foxp3DTR mice were assessed for the production of IFNγ and IL-4. (A) Representative flow cytometric profiles showing staining for CD44 and IFNγ (top) and CD44 and IL-4 (bottom), by CD4+ Foxp3− T cells. Numbers at the top left of each panel represent the percentage of cells in each quadrant. (B-C) The relationship between Treg frequency and (B) IFNγ production or (C) IL-4 production by CD4+CD44hiFoxp3− T cells. (D) The relationship between Treg frequency and the fold-change in IL-4 (◊) and IFNγ (♦) production by CD4+CD44hiFoxp3− T cells (relative to untreated controls). (E) The relationship between Treg frequency and the IL-4+(Th2)/IFNγ+(Th1) ratio. (B-E) Diamonds represent individual mice, and the trend line represents the optimal model for relationship fitting with the use of the least-squares approach. r2 values are the goodness of fit, and P values show significance of a nonzero relationship.
Asymmetric accumulation of Th1 and Th2 cells after titrated Treg depletion. Effector CD4+ T cells from the lymph nodes of DT-treated Foxp3DTR mice were assessed for the production of IFNγ and IL-4. (A) Representative flow cytometric profiles showing staining for CD44 and IFNγ (top) and CD44 and IL-4 (bottom), by CD4+ Foxp3− T cells. Numbers at the top left of each panel represent the percentage of cells in each quadrant. (B-C) The relationship between Treg frequency and (B) IFNγ production or (C) IL-4 production by CD4+CD44hiFoxp3− T cells. (D) The relationship between Treg frequency and the fold-change in IL-4 (◊) and IFNγ (♦) production by CD4+CD44hiFoxp3− T cells (relative to untreated controls). (E) The relationship between Treg frequency and the IL-4+(Th2)/IFNγ+(Th1) ratio. (B-E) Diamonds represent individual mice, and the trend line represents the optimal model for relationship fitting with the use of the least-squares approach. r2 values are the goodness of fit, and P values show significance of a nonzero relationship.
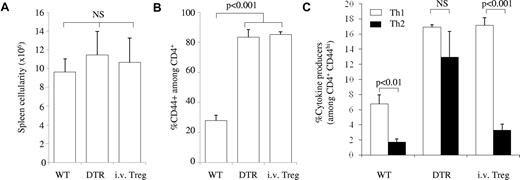
The progressive change in Th2/Th1 ratio as Tregs are removed shows that Tregs not only control the magnitude of T-cell activity but also modulate the “default” effector bias by selectively silencing Th2 cells. To assess the kinetic properties of this selective suppression of the Th2 response, Foxp3DTR Ly5.1 congenic mice were fully depleted of Tregs with a high dose of DT. DT-resistant Tregs were restored to the system on day 7, by the injection of purified CD4+ T cells from Foxp3GFP Ly5.2 mice. Day 7 Treg treatment of Treg-depleted mice had no effect on the magnitude of T-cell activation, as assessed by cellularity (Figure 3A; supplemental Figure 5A) or CD44 expression (Figure 3B; supplemental Figure 5B). When the extent of the Th1 and Th2 responses within the original host cells (Ly5.1+) were measured on day 9, Treg treatment on day 7 was insufficient to reverse the increase in the Th1 response; however, it was strikingly effective at reducing the Th2 response (Figure 3C; supplemental Figure 5C).
Rapid restoration of splenic Th2 suppression by Treg transfer. The magnitude and Th bias were compared for wild-type mice (WT), Foxp3DTR mice injected with DT (DTR), and Foxp3DTR Ly5.1 mice injected with DT (on day 0) and then injected with 5 × 106Ly5.2 Foxp3GFP CD4 T cells 7 days later (i.v.Treg). (A) Splenic cellularity. (B) Proportion of T-cell activation, as measured by CD44 expression on CD4+ T cells. (C) Frequency of CD4+CD44hiFoxp3− T cells producing IFNγ (□) and IL-4 (■). Within the i.v.Treg group, only the responses within the host Ly5.1+ cells were measured; n = 3/group. Error bars indicate SD.
Rapid restoration of splenic Th2 suppression by Treg transfer. The magnitude and Th bias were compared for wild-type mice (WT), Foxp3DTR mice injected with DT (DTR), and Foxp3DTR Ly5.1 mice injected with DT (on day 0) and then injected with 5 × 106Ly5.2 Foxp3GFP CD4 T cells 7 days later (i.v.Treg). (A) Splenic cellularity. (B) Proportion of T-cell activation, as measured by CD44 expression on CD4+ T cells. (C) Frequency of CD4+CD44hiFoxp3− T cells producing IFNγ (□) and IL-4 (■). Within the i.v.Treg group, only the responses within the host Ly5.1+ cells were measured; n = 3/group. Error bars indicate SD.
Cellular and molecular basis for Treg control over the magnitude and bias of the effector response
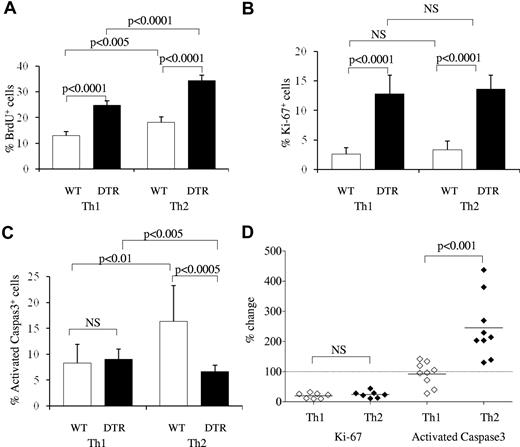
The rapid and specific collapse of Th2 responses within 2 days of Treg reconstitution provides an independent demonstration of asymmetric regulation of Th1 and Th2 cells by Tregs and shows distinct kinetic patterns for Treg control over magnitude and effector bias. To investigate the cellular mechanisms by which Tregs have this asymmetric influence, the proliferation and apoptosis of Th1 and Th2 subsets were measured in the presence and absence of Tregs in vivo. Proliferation was measured with both BrdU incorporation (Figure 4A) and Ki67 expression (Figure 4B). Proliferation rates of effector T cells in a wild-type host were low, with little to no difference detected between the Th1 and Th2 subsets. After complete Treg depletion for 9 days, the percentage of cells showing evidence of proliferation rose substantially among both subsets: ∼ 2-fold by BrdU incorporation and ∼ 5-fold by Ki67 expression (Figure 4A-B). These data are consistent with the role Tregs play in suppressing the magnitude of immune responses; however, they are not sufficient to explain the Th1 bias caused by Tregs, because both Th1 and Th2 were affected equally.
Asymmetric induction of Th1 versus Th2 apoptosis by Tregs. The effect of Tregs on the proliferation and apoptosis of Th1 and Th2 cells was determined by measurement of BrdU incorporation, Ki67 expression, and activated caspase 3 expression within IL-4– and IFNγ-producing cells in the lymph nodes of wild-type and DT-treated Foxp3DTR mice. (A) Wild-type and DT-treated Foxp3DTR mice were treated daily with BrdU from the time of DT administration, and incorporation was measured in IFNγ-expressing Th1 cells and IL-4–expressing Th2 cells (n = 5/group). (B) Ki67 expression in Th1 and Th2 subsets of wild-type and DT-treated Foxp3DTR mice (n = 9, 11). (C) Activated caspase 3 expression in Th1 and Th2 subsets of wild-type and DT-treated Foxp3DTR mice (n = 9, 11). (D) The effect of Tregs on Th1 and Th2 subsets, measured by calculating the percentage change in Ki67 and activated caspase 3 expression in wild-type versus DT-treated Foxp3DTR mice. Each diamond represents an individual wild-type mouse (n = 9), normalized to the average value of DT-treated Foxp3DTR mice (n = 11). Error bars indicate SD.
Asymmetric induction of Th1 versus Th2 apoptosis by Tregs. The effect of Tregs on the proliferation and apoptosis of Th1 and Th2 cells was determined by measurement of BrdU incorporation, Ki67 expression, and activated caspase 3 expression within IL-4– and IFNγ-producing cells in the lymph nodes of wild-type and DT-treated Foxp3DTR mice. (A) Wild-type and DT-treated Foxp3DTR mice were treated daily with BrdU from the time of DT administration, and incorporation was measured in IFNγ-expressing Th1 cells and IL-4–expressing Th2 cells (n = 5/group). (B) Ki67 expression in Th1 and Th2 subsets of wild-type and DT-treated Foxp3DTR mice (n = 9, 11). (C) Activated caspase 3 expression in Th1 and Th2 subsets of wild-type and DT-treated Foxp3DTR mice (n = 9, 11). (D) The effect of Tregs on Th1 and Th2 subsets, measured by calculating the percentage change in Ki67 and activated caspase 3 expression in wild-type versus DT-treated Foxp3DTR mice. Each diamond represents an individual wild-type mouse (n = 9), normalized to the average value of DT-treated Foxp3DTR mice (n = 11). Error bars indicate SD.
We therefore hypothesized that differential induction of apoptosis may explain the Th-biasing effect. To measure the effect of Tregs on the apoptosis of Th1 and Th2 cells, cells from wild-type or DT-treated Foxp3DTR mice were assessed for expression of activated caspase 3, which sensitively marks cells in the early stages of apoptosis.30 There was no significant change in the proportion of Th1 cells expressing activated caspase 3 in wild-type versus DT-treated Foxp3DTR mice (Figure 4C). However, in wild-type mice the proportion of Th2 cells staining positive for activated caspase 3 was 2-fold higher than that of Th1 cells, and in mice depleted of Tregs the proportion of apoptotic Th2 cells dropped to below that of Th1 cells. With the use of Treg-depleted mice as a control index, the effector of Tregs on Th1 and Th2 subsets can be enumerated at an 80% reduction in proliferation for both subsets and a Th2-specific 150% increase in apoptosis (Figure 4D). Effects of similar magnitude were observed in the spleen (supplemental Figure 6). We conclude that the lower frequency of Th2 versus Th1 cells in wild-type mice correlates with an enhanced rate of Th2 cell apoptosis that is because of the presence of Tregs.
A candidate for the molecular mechanism of Treg-mediated heightened suppression of Th2 responses was CTLA-4, because of the sharp increase in IgE and autoAbs that develops when CTLA-4 is specifically removed from Tregs13 and the autoimmunity caused by apoptosis-resistant Th2 cells in CTLA-4 knockout mice.31,32 To determine whether CTLA-4 was sufficient, in the absence of Tregs, to restore the Th1 bias observed in wild-type mice, we treated Treg-depleted mice with the recombinant fusion protein CTLA4-Ig which, like endogenous CTLA-4, is known to bind B7 molecules with high affinity and to prevent T-cell activation.33 Treatment with CTLA4-Ig either during initial priming (day 0 of Treg-depletion) or during reaction expansion (day 5 or day 7) reduced IL-4 production to a greater extent than IFNγ production (supplemental Figure 7A), returning the Th2/Th1 ratio to one approaching the wild-type context (supplemental Figure 7B). Because day 0 treatment may interfere with initial priming34 and day 7 treatment had reduced effectiveness, treatment on day 5 was analyzed in greater detail. CTLA4-Ig treatment on day 5 of DT treatment had no effect on the magnitude of the Th1 response at day 9 but sharply limited Th2 expansion (Figure 5A). The net effect of CTLA4-Ig was to return the Th2/Th1 ratio from 1:1 in Treg-deficient mice to the “wild-type” ratio of 5:1 (Figure 5B). Notably, as observed with the Treg-reconstitution experiment, injection of CTLA4-Ig on day 5 kinetically separated the “magnitude control” and “Th bias” regulatory effects. Further supporting the parsimonious conclusion that Treg suppression of Th2 responses operates through CTLA-4 was the effect of CTLA4-Ig on apoptosis. Compared with the Treg-depleted state, CTLA4-Ig treatment in Treg-depleted mice had no effect on Th1 apoptosis but significantly increased Th2 apoptosis to levels approaching that of Treg-sufficient mice (Figure 5C).
CTLA4 and anti–IL-2 can substitute for Tregs in restoring effector T-cell bias. DT-treated Foxp3DTR mice were treated with CTLA4-Ig on day 5 or anti–IL-2 Ab on days 0-8. (A) Frequency of IL-4 and IFNγ producers among CD4+CD44hiFoxp3− T cells and (B) Th2/Th1 ratio in wild-type mice, DT-treated Foxp3DTR mice, DT-, and CTLA4-Ig–treated Foxp3DTR mice and DT- and anti–IL-2-treated Foxp3DTR mice, after 9 days of treatment with DT. All data are from the spleen (n = 3-4/group). (C) The effect of CTLA4-Ig or anti–IL-2 Ab on apoptosis in Th1 and Th2 subsets in the absence of Tregs, measured by calculating the percentage change in activated caspase 3 expression in DT-treated mice (diamonds), DT-treated CTLA4-Ig–treated mice (squares) or DT-treated anti–IL-2-treated mice (circles), compared with wild-type mice (dashed line at 100%). Each symbol represents an individual mouse, P values represent significant differences in CTLA4-Ig– or anti–IL-2 Ab-treated mice versus Treg depletion alone. Error bars indicate SD.
CTLA4 and anti–IL-2 can substitute for Tregs in restoring effector T-cell bias. DT-treated Foxp3DTR mice were treated with CTLA4-Ig on day 5 or anti–IL-2 Ab on days 0-8. (A) Frequency of IL-4 and IFNγ producers among CD4+CD44hiFoxp3− T cells and (B) Th2/Th1 ratio in wild-type mice, DT-treated Foxp3DTR mice, DT-, and CTLA4-Ig–treated Foxp3DTR mice and DT- and anti–IL-2-treated Foxp3DTR mice, after 9 days of treatment with DT. All data are from the spleen (n = 3-4/group). (C) The effect of CTLA4-Ig or anti–IL-2 Ab on apoptosis in Th1 and Th2 subsets in the absence of Tregs, measured by calculating the percentage change in activated caspase 3 expression in DT-treated mice (diamonds), DT-treated CTLA4-Ig–treated mice (squares) or DT-treated anti–IL-2-treated mice (circles), compared with wild-type mice (dashed line at 100%). Each symbol represents an individual mouse, P values represent significant differences in CTLA4-Ig– or anti–IL-2 Ab-treated mice versus Treg depletion alone. Error bars indicate SD.
The involvement of CTLA-4 in enhanced Th2 regulation may be explained by the effect of Treg CTLA-4 expression on the tonic dampening of CD80/CD86 expression on APCs,13 because anti-CD28 blockade sharply reduces the Th2 response through a disproportionate and irreplaceable role for IL-2 on Th2 differentiation.35 This model would imply that IL-2 neutralization during Treg depletion would limit the Th2 response, similar to CTLA4-Ig provision. This was tested through the treatment of DT-treated Foxp3DTR mice with neutralizing anti–IL-2 Ab. IL-2 neutralization from day 0 of Treg depletion was unable to limit the size of the day 9 Th1 response but was, by contrast, highly effective in limiting the Th2 response (Figure 5A). As with CTLA4-Ig, this restored the Th2/Th1 ratio back to the Th1 bias observed in wild-type mice (Figure 5B) and was associated with a restoration of wild-type levels of Th2 apoptosis (Figure 5C).
Discussion
These results show an asymmetric regulation of Th cells by Tregs. Tregs not only have a greater in vivo suppressive effect on the Th2 response than the Th1 response but also have a more complex relationship. These data indicate that suppression of Th1 responses by Tregs operates along simple principles, with frequency-proportional suppression. By contrast, Th2 suppression by Tregs is disproportionate to frequency, with even moderate Treg numbers capable of efficiently quenching a Th2 response. The model generated here suggests that the simple proportional component of Treg suppression is mediated by a reduction in effector T-cell proliferation, whereas the heightened suppression of Th2 responses is associated with the additional effect of enhanced apoptosis in the presence of Tregs (Figure 6). Because the Th1 bias and Th2 apoptotic effect generated by Tregs can be replicated through CTLA4-Ig (Figure 5) and loss of CTLA-4 on Tregs results in Th2-associated autoimmune disease,13 CTLA-4 expression on Tregs is a probable mediator of this asymmetric behavior. The involvement of CTLA-4 in enhanced Th2 regulation may be explained by the effect of Treg CTLA-4 expression on the tonic dampening of CD80/CD86 expression on APCs,13 because anti-CD28 blockade sharply reduces the Th2 response through a disproportionate and irreplaceable role for IL-2 on Th2 differentiation,35 a trait which is shared with Tregs.36 This model is also consistent with the reversal of hyper IL-4 production in Treg-deficient Foxp3sf mice crossed onto the CD28-deficient or IL-2–deficient backgrounds.34,37 In addition to the indirect effect of Tregs on IL-2 levels, by CTLA-4–mediated reductions in T-cell priming, Tregs have been shown to directly reduce IL-2 availability by enhanced cytokine consumption38 and trans-reduction in IL-2 signaling capacity.39 Pandiyan et al38 demonstrated that the IL-2 consumption mechanism increases T-cell apoptosis, the results shown here suggest that this effect is primarily directed toward the Th2 lineage.
Asymmetric control over immune activation by Tregs. Tregs have 2 key effects on controlling Th1 and Th2 responses. First, Tregs inhibit the proliferation of both subsets, reducing the magnitude of the immune response. Second, Tregs specifically enhance the apoptosis of Th2 cells by CTLA4 activity and reductions in IL-2 bioavailability. The net effect of these 2 forms of regulation is to shift the Th2/Th1 balance from being at parity in the absence of Tregs to heavily Th1 skewed in the presence of Tregs.
Asymmetric control over immune activation by Tregs. Tregs have 2 key effects on controlling Th1 and Th2 responses. First, Tregs inhibit the proliferation of both subsets, reducing the magnitude of the immune response. Second, Tregs specifically enhance the apoptosis of Th2 cells by CTLA4 activity and reductions in IL-2 bioavailability. The net effect of these 2 forms of regulation is to shift the Th2/Th1 balance from being at parity in the absence of Tregs to heavily Th1 skewed in the presence of Tregs.
These data build on recently emerging evidence that Tregs use distinct molecular mechanisms to control effector T-cell subsets, with T-bet-, IRF4-, Stat3-, and Bcl6-deficient Tregs exhibiting poor control over Th1, Th2, Th17, and Tfh subsets, respectively, while leaving the remainder subsets unchanged.9-11,14 Various cellular mechanisms have been invoked to explain these subset-specific activities. For example, T-bet is up-regulated on a subset of Tregs during Th1 responses and is probably driven by Th1-mediated IFNγ production because up-regulation occurs by IFNγR and Stat6 signaling.9 Unlike T-bet, Stat3 is expressed by most Tregs. However, activation of Stat3 depends on the appropriate cytokine signal, such as the Th17 cytokine IL-6, making it probable that enhanced Th17-regulation by Tregs is induced by a Th17 microenvironment.10 In the case of IRF4, it is not clear whether IRF4 is expressed by a subset of Tregs or by the whole population, or whether the Th2-specific regulatory function is constitutive or activated by the presence of Th2 cytokines10 ; however, it has been shown that IRF4 directly regulates Blimp1, in turn regulating IL-10 and CCR6.40 Bcl6, by contrast, marks a transient inducible population of Tregs, which colocalize with Tfh cells in active germinal centers.14 Currently, there is no evidence to suggest that these functional regulators (T-bet, Stat3, IRF4, Blimp, Bcl6) represent stable Treg subsets. Indeed, evidence suggests that these are transient transcriptional pathways, activated in response to microenvironmental stimuli to allow colocalization with effector T cells and silenced after the termination of the initiating stimuli.14
The effect that we show here is distinct from the subset-specific regulatory pathways shown by Treg-specific deficiency of T-bet, IRF4, Stat3, or Bcl6. Loss of each of these genes in Tregs results in qualitative changes in Treg function without any quantitative decrease.9-11,14 By contrast, the use of DT to titrate Treg number introduces a quantitative change, without any qualitative genetic manipulation, leaving all the effector-specific response pathways intact. The finding that Treg suppression of effector T-cell subsets relies on qualitatively different molecular mechanisms did not predict the finding here that shifts in subset suppression efficiency change with quantitative Treg frequency. However, the reverse is true, because the behavior shown by Treg titration implies molecular stratification of the suppression of different subsets, because if Tregs used the same molecular mechanisms working with equal efficiency against different subsets, asymmetric quantitative effects would not occur. The result is a model whereby Tregs use qualitatively different systems of suppression for different effector T-cell subsets, which differentially titrate in a quantitative manner because of intrinsic variation in the response of effector T-cell subsets.
Many studies have attempted to correlate Treg frequency with disease state.41 It is therefore important to develop a theoretical basis for understanding the effects that quantitative changes in Treg frequency can have on disease susceptibility. Under the model derived from the empirical data of the titration curve, the effect of quantitative decreases in Treg number is constant for Th1 responses but disproportionate for Th2 responses. In the current study we have focused on the purely quantitative effects of Tregs on Th bias; however, as shown by the Unmodulated Card11 mutation,42 qualitative effects on Tregs are also able to release a strong Th2 response from Treg suppression. In the human disease context, polymorphisms altering number and function would be expected to interact to produce a spectrum of Treg functionality, which may operate akin to the titrated frequency curve in the experimental system here. If future studies show a similar relationship in humans, the results would have large implications for the potential effects of Treg therapy.
Finally, in addition to showing changes in Th bias associated with quantitative changes in Treg number, these studies suggest a molecular basis for therapeutic intervention. The ability of long-term CTLA4-Ig treatment to blockade T-cell activation and autoimmune disease is well established, with efficacious results in rheumatoid arthritis and ongoing trials in type 1 diabetes, ulcerative colitis, and lupus.43 Here, we demonstrated an additional capacity of CTLA4-Ig to induce Th2 apoptosis and to rapidly restore the Th2/Th1 ratio in the absence of Treg cells. Although immunodysregulation polyendocrinopathy enteropathy X-linked syndrome, caused by Treg absence, is a serious but rare condition, more common autoimmune diseases may benefit from restoration of the Th2/Th1 ratio. A study on patients with rheumatoid arthritis found reduced CTLA-4 expression on Tregs, associated with reduction suppression capacity.44 The presence of this phenotype in patients seropositive for rheumatoid arthritis but not patients seronegative for psoriatic arthritis44 raises the potential scenario that reduced CTLA-4 expression on Tregs releases autoreactive Th2 cells. Because CTLA4 is a candidate susceptibility gene for rheumatoid arthritis, type 1 diabetes, systemic lupus erythematosus, celiac disease, Graves disease, and Hashimoto thyroiditis,45-48 partial CTLA-4 deficiency on Tregs may be a common contributor to multiple diseases. These studies suggest that such a defect may translate to disproportionate autoreactive Th2 activation and raise the potential that CTLA4-Ig treatment may prove a rapid and efficacious treatment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Rudensky for providing Foxp3DTR mice and J. Kim and S. Lesage for constructive advice. They also thank S. Schonefeldt for mouse colony support.
This work was supported by grants from the VIB and JDRF (A.L.).
Authorship
Contribution: L.T., J.D., and D.F. performed the experiments; L.E.M. performed statistical analysis; L.T., J.A.A., M.C.C., C.C.G., and A.L. designed the experiments; and L.T. and A.L. wrote the manuscript.
Conflict-of-interest disclosure: L.E.M. is currently employed at UCB. The remaining authors declare no competing financial interests.
Correspondence: Adrian Liston, VIB and University of Leuven, Leuven 3000, Belgium; e-mail: adrian.liston@vib.be.