Abstract
The safety and efficacy of weekly rituximab 375 mg/m2 (×4), given within 3 days of acute TTP admission, with standard therapy (PEX and steroids) was evaluated. Clinical outcomes were compared to historical controls (n = 40) who had not received rituximab. Within the trial group, 15 of 40 required ICU admission and 15% of all cases with the highest troponin T levels on admission were ventilated. Before the second rituximab infusion, 68% of cases had a platelet count > 50 × 109/L and 38% > 150 × 109/L. Fewer PEX were required in whites compared to nonwhite in the rituximab group (mean 14 vs 21, P = .0095). Inpatient stay was reduced by 7 days in the non-ICU trial cases compared to historical controls (P = .04), especially in whites, with a mean reduction of 7 days (P = .05). Ten percent of trial cases relapsed, median, 27 months (17-31 months), compared to 57% in historical controls, median 18 months (3-60 months; P = .0011). There were no excess infections or serious adverse events with rituximab. In conclusion, rituximab appears a safe and effective therapy. Inpatient stay and relapse are significantly reduced in the rituximab cohort. Rituximab should be considered in conjunction with standard therapy on acute presentation of TTP. This study was registered at www.clinicaltrials.gov as NCT009-3713.
Introduction
Acquired thrombotic thrombocytopenic purpura (TTP) is an acute, life-threatening illness characterized by thrombocytopenia, microangiopathic hemolytic anemia (MAHA), and often signs of organ dysfunction—typically neurologic, cardiac, renal, or abdominal symptoms. In the majority of cases, there is no obvious underlying precipitant, although a genetic link has been identified.1 Severe deficiency of ADAMTS13 is present in most cases of acquired TTP because of the presence of antibody, primarily IgG, to ADAMTS13. The mainstay of treatment remains plasma exchange (PEX) and steroids.2 However, patients with acquired idiopathic autoimmune disease are more likely to take longer to achieve a normal platelet count and require more plasma therapy,3 with a relapse rate of 30%-50%.4,5 In such refractory/relapsing TTP patients, further immunosuppressive therapy may be required, such as Ciclosporin, cyclophosphamide, or vincristine. Increasingly, rituximab is being reported for the treatment of acute acquired idiopathic TTP,6-8 but only in small case series and anecdotal reports. We present the results of the first phase 2 trial of the use of rituximab, in conjunction with standard therapy (PEX and steroids) within 3 days of admission for acute TTP. The primary aim was to determine the safety and efficacy of rituximab. We also sought to determine whether using rituximab at presentation shortened the time to remission and prevented end organ damage due to microvascular thrombi.
Methods
This was a multicenter study in the United Kingdom involving the South East England TTP study group. Adults over the age of 18 years presenting with de novo or relapsed acute TTP were eligible for entry into the trial. The diagnosis was based on thrombocytopenia, microangiopathic hemolytic anemia, normal clotting screen, and increased lactate dehydrogenase (LDH) to one and a half times the normal upper limit. The trial was approved by the ethics committees of all participating sites, and all human participants gave written informed consent in accordance with the Declaration of Helsinki. In unconscious patients or those unable to consent, consent was obtained in accordance with current regulatory requirements (next of kin or patient advocate; www.clinicaltrial.gov/NCT00937131. MREC: 08/H0810/54).
A total of 40 patients with a diagnosis of acute TTP were entered between 2006 and 2009. and follow-up was for at least 12 months after admission. The historical controls were patients from the South East England registry from the participating hospitals involved with the trial, and were matched, as far as possible, according to sex, ethnicity, and number of relapses to the trial group. None of the historic patients reviewed had received rituximab.
Study design and methodology
This was a nonrandomized trial in patients with acute TTP. All patients received PEX using solvent detergent (S/D) FFP (Octaplas; Octapharma) continued daily from admission and until a sustained platelet count of > 150 × 109/L was reached for 2 consecutive days. Steroids were given as per the local protocol, typically 1 g of methylprednisolone intravenously daily for the first 3 days from admission. Historical control patients similarly followed the British Committee for Standards in Haematology guidelines.2 Cryosupernatant and Octaplas were also available before January 2006.
Patients fulfilling the inclusion criteria received rituximab 375 mg/m2 intravenously within the first 3 days of admission and diagnosis. Because PEX is the primary treatment modality, symptomatic patients, such as those with new/progressive neurologic or cardiac symptoms, were given PEX at increased frequency (twice a day). This was continued as clinically indicated with a minimum of 4 hours between infusion and further PEX therapy. All patients received premedication rituximab as per the local protocol, and typically antihistamine (eg, 10 mg of IV Piriton) and Paracetamol. Hydrocortisone was deemed unnecessary before the first infusion because the patients had received steroids and were given subsequent doses if there were previous cytokine-mediated reactions. A total of 4 treatments, 1 per week for 4 weeks, were given. In patients whose ADAMTS13 levels remained below the normal range (55%-126% by collagen-binding assay), or those with persistently detectable anti-ADAMTS13 IgG antibodies, further rituximab was given, up to 8 infusions. This was typically determined during outpatient follow-up after completing the first 4 treatments.
Exclusion criteria
All pregnant or breastfeeding females, HIV-positive patients (tested preconsent), and those who had had childhood TTP (< 18 years), with hemolytic uremic syndrome, with diarrhea positively or negatively associated with acute renal failure, or transplant-associated thrombotic microangiopathy were excluded. Patients with concurrent malignancies, with the exception of appropriately treated localized epithelial or cervical cancer, were excluded. Patients with a history of tumors currently in remission were included.
Objectives
The objective of the study was to determine the safety, efficacy, and tolerability of rituximab in combination with PEX in patients with acute idiopathic TTP. The primary efficacy outcome measure was the number of PEX treatments to remission as defined by the number of procedures per admission compared with historical controls.
Secondary efficacy outcome measures were length of stay, mortality at 3 months, B-cell depletion as measured by CD19 count, effect on ADAMTS13 activity and anti-ADAMTS13 IgG, and time to relapse. The main safety outcome measures were infusion-associated reactions, allergic reactions, immunoglobulin levels, and frequency of infections at 12 months of follow-up.
Remission was defined as sustained platelet count > 150 × 109/L for 2 consecutive days. Relapse was defined as readmission with thrombocytopenia (< 150 × 109) with or without new symptoms 30 days after discharge from an acute episode. Exacerbation was defined as thrombocytopenia within 30 days of discharge.9
Assays
Plasma ADAMTS13 activity was measured using a collagen-binding method.10,11 ADAMTS13 activity was expressed as a percentage relative to the activity in pooled normal plasma (normal range 55%-126%; lower limit of detection 5%).
Anti-ADAMTS13 IgG was measured using an ELISA technique.8,12 Microtiter plates were coated with rADAMTS13 (Baxter Bioscience), patient plasma was added and incubated for 1 hour, and any anti-ADAMTS13 IgG present was detected using anti–human globulin. A standard curve was prepared by diluting an index reference plasma in PBS/BSA to achieve 100%, 80%, 40%, 20%, 10%, 5%, and 0% concentrations. The normal range was < 6.1% calculated as the 95th percentile of 49 normal healthy controls.
CD19, a marker of peripheral blood B lymphocytes, was determined using CD19-FITC (Beckman Coulter).13 Serial CD19 measurements were performed before each rituximab therapy and at follow-up visits to assess the degree of B-cell depletion and recovery time to determine whether there was any association between infection risk and the return of CD19 levels to within the normal range (5%-15%) and relapse.
Statistical analysis
Sample size for days to remission.
Using standard methods for a 2-sample t test using the log-transformed data, the sample size required to detect this difference with 90% power and 5% significance was 34 in each group. We planned to recruit 40 patients, allowing for a 20% dropout rate or loss to follow-up.
Sample size for number of plasma exchanges.
Using standard methods for a 2-sample t test using the log-transformed data, the sample size required to detect this difference with 90% power and 5% significance was 19 in each group.
General statistical methods.
Where data were shown to be normally distributed, 2-sample t tests were used to assess differences between groups. If data were demonstrably nonparametric, we used log transformations of datasets and performed 2-sample t tests. Where appropriate, a nonparametric alternative to the t test, the Mann-Whitney U test, was used. Time to relapse was compared using Kaplan-Meier estimates for right-censored data, and the log-rank test was used to assess differences between trial and historic patient cohorts.
Results
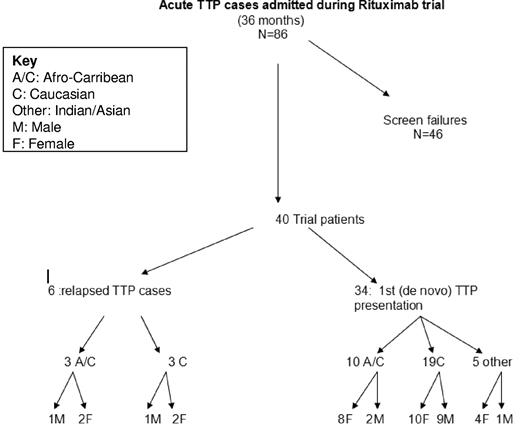
During the 36-month trial period, 86 consecutive patients were admitted (Figure 1). Of the 46 patients who failed to meet the inclusion criteria, there were 7 deaths within 24 hours of admission, accounting for 15% of the total screen failure group. Approximately one-third of this group received rituximab off-trial and their results were not included. Nine of these patients were considered too late for entry as determined by the inclusion criteria and 4 patients were adolescents (< 18 years), again failing to meet entry criteria. A further 15% were found to have a diagnosis other than TTP on admission. Two patients declined study entry, and 1 of these subsequently received rituximab off-trial for refractory TTP (Table 1).
Characteristics of acute TTP patients admitted to the rituximab trial. Summary of all the patients screened for entry into the trial over a 36-month period, including ethnicity and identification of de novo or relapsed disease.
Characteristics of acute TTP patients admitted to the rituximab trial. Summary of all the patients screened for entry into the trial over a 36-month period, including ethnicity and identification of de novo or relapsed disease.
Screen failures for entry into rituximab trial*
| Admission . | Number . | Received rituximab off-trial . |
|---|---|---|
| Died within 24 h of admission | 7 | N/A |
| Secondary TTP (eg, pregnancy, HIV, pancreatitis) | 14 | 1 |
| Other TMAs† | 10 | |
| Declined trial entry | 2 | 1 |
| Failed to meet criteria | 13 | 12 |
| Admission . | Number . | Received rituximab off-trial . |
|---|---|---|
| Died within 24 h of admission | 7 | N/A |
| Secondary TTP (eg, pregnancy, HIV, pancreatitis) | 14 | 1 |
| Other TMAs† | 10 | |
| Declined trial entry | 2 | 1 |
| Failed to meet criteria | 13 | 12 |
TTP indicates thrombocytopenic purpura; TMA, thrombotic microangiopathy; and N/A, not applicable.
Patients failing to meet the entry criteria included those < 18 years, failure to enroll within 3 days as per protocol, and referral due to refractory disease. A total of 46 cases failed inclusion criteria.
Other TMAs included atypical hemolytic uremic syndrome, malignant hypertension, acute systemic sclerosis, carcinoma, bacterial endocarditis, and herpes simplex encephalitis.
Of the cohort of 40 trial patients, 34 were a de novo presentation and 6 patients were a TTP relapse. Within the trial group, there were 3 deaths and 4 relapses, 3 of which were successfully retreated with rituximab. One patient in the trial group had previously received rituximab, but this was more than a year before entry into this study. One patient was withdrawn from the study and subsequently was found to have an alternative diagnosis.
Patient characteristics
Within the trial group, the female to male ratio was 2:1. More than half of the group were white and one-third were Afro-Caribbean. The ratio of female-to-male white patients was 12:10, whereas for Afro-Caribbean and Indian or Asian patients, this ratio was approximately 3:1. All 3 deaths in the rituximab-treated patients were white males, and this occurred 11, 15, and 25 days after admission, with 1 of these patients previously receiving rituximab treatment.
On admission, 15 of the patients required admission to the ICU. Six patients were intubated and ventilated (15% of the total trial cohort). Seventy-seven percent of the patients presented with neurologic features (headache, stroke, coma, and seizures), 47% had fever (temperature > 37.5°C), 47% had abdominal symptoms, and 47% had increased troponin T or cardiac symptoms. Forty-two percent of the patients had renal impairment that normalized with treatment (Table 2).
Admission characteristics and laboratory parameters of trial patients and historical controls*
| . | Trial patients . | Historical control patients . | P . |
|---|---|---|---|
| Female:male | 26:14 | 33:7 | 1.0 |
| De novo: relapsed | 34:6 | 31:9 | 1.0 |
| Ethnicity | 22 White (12 F, 10 M) | 26 White (22 F, 4 M) | |
| 13 A/C (10 F, 3 M) | 11 A/C (9 F, 2 M) | ||
| 5 Other (4 F, 1 M) | 3 Other: (2 F, 1 M) | ||
| Median age (range) | 42 (21-76) | 42 years (18-78 years) | .4 |
| Symptoms | Neurologic: 31 of 40 | Neurologic: 29 of 40 | .09 (including cardiac cases) |
| Fever: 19 of 40 | Fever: 15 of 40 | .2 (excluding cardiac cases) | |
| Renal: 17 of 40 | Renal: 10 of 38 | ||
| Abdominal: 19 of 40 | Abdominal: 14 of 38 | ||
| Cardiac: 19 of 40 | Cardiac: 4 of 37† | ||
| Median Hb, g/d L (range) | 8.6 (3.7-13.8) | 8.5 (4.8-15) | .7 |
| Median platelets, ×109/L (range) | 13 (5-60) | 14 (4-84) | .7 |
| Median LDH, IU (range) | 2088 (498-4565) | 1724 (274-5042) | .7 |
| Median bilirubin, μmol/L, day 1 (range) | 51 (9-141) | 46 (8-179) | .8 |
| Median creatinine, μmol/L (range) | 94 (52-353) | 94 (61-516) | .8 |
| Median ADAMTS13 activity, % (range) | < 5% (< 5%-32%) | < 5%(< 5%-40%) | .97 |
| Median anti-ADAMTS13 IgG, % (range) | 40% (6%-162%) | 78% (8%-140%) | .25 |
| . | Trial patients . | Historical control patients . | P . |
|---|---|---|---|
| Female:male | 26:14 | 33:7 | 1.0 |
| De novo: relapsed | 34:6 | 31:9 | 1.0 |
| Ethnicity | 22 White (12 F, 10 M) | 26 White (22 F, 4 M) | |
| 13 A/C (10 F, 3 M) | 11 A/C (9 F, 2 M) | ||
| 5 Other (4 F, 1 M) | 3 Other: (2 F, 1 M) | ||
| Median age (range) | 42 (21-76) | 42 years (18-78 years) | .4 |
| Symptoms | Neurologic: 31 of 40 | Neurologic: 29 of 40 | .09 (including cardiac cases) |
| Fever: 19 of 40 | Fever: 15 of 40 | .2 (excluding cardiac cases) | |
| Renal: 17 of 40 | Renal: 10 of 38 | ||
| Abdominal: 19 of 40 | Abdominal: 14 of 38 | ||
| Cardiac: 19 of 40 | Cardiac: 4 of 37† | ||
| Median Hb, g/d L (range) | 8.6 (3.7-13.8) | 8.5 (4.8-15) | .7 |
| Median platelets, ×109/L (range) | 13 (5-60) | 14 (4-84) | .7 |
| Median LDH, IU (range) | 2088 (498-4565) | 1724 (274-5042) | .7 |
| Median bilirubin, μmol/L, day 1 (range) | 51 (9-141) | 46 (8-179) | .8 |
| Median creatinine, μmol/L (range) | 94 (52-353) | 94 (61-516) | .8 |
| Median ADAMTS13 activity, % (range) | < 5% (< 5%-32%) | < 5%(< 5%-40%) | .97 |
| Median anti-ADAMTS13 IgG, % (range) | 40% (6%-162%) | 78% (8%-140%) | .25 |
A/C indicates Afro-Caribbean.
Shown is a comparison of cases and controls with no evidence of statistical difference between all the categories highlighted other than cardiac (due to the unavailability/testing of troponin levels).
There were fewer cardiac cases because troponin T levels were not available throughout this period.
Management
Thirty-two patients received at least 4 rituximab treatments as per the protocol. Two patients received 2 treatments (1 was withdrawn from the study and 1 died) and 2 other patients received 3 treatments (both died before completing the rituximab course). Four patients required 6 treatments in total, and 2 patients required 8 treatments in total. Based on ADAMTS13 assay results, the decision was made after 4 infusions to give further rituximab; these 6 patients were all nonwhite. There was no correlation between severity of presentation, based on patients requiring admission to ICU at presentation—and in particular intubation and ventilation on admission—and the number of additional doses of rituximab required. Thirty-eight patients received steroids on admission, typically pulsed methylprednisolone, and 4 patients also received additional vincristine in conjunction with rituximab during their acute episode.
Laboratory parameters
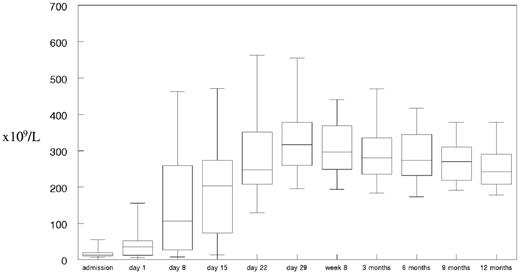
Before the second rituximab infusion, 68% of patients had a platelet count > 50 × 109/L and 38% > 150 × 109/L (Figure 2). The median time to sustained normalization of the platelet count, including the patients admitted into intensive care, was 12 days. A summary of the laboratory parameters for the trial group can be found in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Hemoglobin and reticulocytes took between 29 days and 8 weeks to normalize despite patients having completed therapy with PEX and rituximab. Bilirubin and LDH levels were typically normal before day 8 (before the second rituximab infusion). There were no cases of acute anuric/oliguric renal failure. Troponin T measurements on admission were > 0.05μM (normal range < 0.01μM) in 14 of 30 patients documented. The highest markers of cardiac damage were seen in the 6 patients requiring intubation and ventilation on admission. Eleven of the 14 patients with increased troponin T levels were admitted to the ICU on admission
Platelet count in trial patients receiving rituximab during admission and up to 1 year of follow-up. Median platelet count in a box-whisper format from admission, before each rituximab infusion and at follow-up time points as defined for the trial group.
Platelet count in trial patients receiving rituximab during admission and up to 1 year of follow-up. Median platelet count in a box-whisper format from admission, before each rituximab infusion and at follow-up time points as defined for the trial group.
Historical controls
Forty controls, who were matched as far as possible for sex, ethnicity, and de novo presentation or relapsed disease and who did not received rituximab during admission, were included in the trial. These patients were from the South East England registry and were chosen by the completeness of their data. A summary of patient characteristics and laboratory parameters are given in Table 2. Thirty-five patients received steroids on admission. Other therapies used to achieve remission were Ciclosporin (n = 7), defibrotide (n = 5), cyclophosphamide (n = 3), splenectomy (n = 1), and vincristine (n = 9) in 15 patients. There were 3 deaths in the historical group, 2 during the presenting admission and 1 on relapse. Twenty-one patients had an acute TTP relapse a median of 18 months (3-60 months) after this documented admission. Two patients subsequently received rituximab in remission to prevent further TTP episodes based on reduced ADAMTS13 activity (< 5%) and the presence of anti-ADAMTS13 IgG antibodies.
Number of plasma exchanges
In the historic group, the median number of PEX treatments until remission were 18 (range, 6-92). Categorization of first-episode-only patients according to ethnicity, the median number of PEX treatments to remission for whites was 17 (range, 6-92), for Afro-Caribbean patients it was 17 (range, 12-40), and for Indian/Asian patients it was 19 (range, 15-21).
In the trial group, the median number of PEX treatments until remission was 16.5 (range, 4-34). Although less than the historical controls, it did not reach statistical significance (P = .5 by Mann Whitney test). Divided into ethnicity and only including those with de novo presentation in the trial group, for Afro-Caribbean patients, the median number of PEX treatments until remission was 24 (range, 6-34), for white patients, 11.5 (range, 4-30), and for Indian/Asian patients, 23 (range, 6-34). The number of PEX treatments until remission in the white patients was significantly less than that for nonwhite patients receiving rituximab (mean 14 vs 21; P = .0095), suggesting a relevant ethnic difference.
Number of days admitted
There was no difference in the median number of days admitted between patients (16.5; range, 5-49) and historic controls (20; range, 5-62; P = nonsignificant). However, when ICU patients were excluded from the rituximab trial, there was a statistically significant reduction in in-patient days in those who received rituximab (mean, 7 days; P = .04). The reduction in the number of days admitted in the white trial patients compared with historical white controls was also significant (mean 16 vs 23 days, difference of 7 days; P = .05).
CD19
The presenting CD19 levels of the total lymphocyte count were 23% (range, 2.6%-39.90%; normal range, 5%-15%) on admission and before the first rituximab infusion were 21% (range, 10.7%-51.1%). There was a significant progressive decrease in CD19 between the first (1.4%; range, 0%-42%) and second (0.97%; range, 0%-5.43%) rituximab treatment. Before the fourth rituximab treatment, CD19 was 0.5% (range, 0%-2.78%; Figure 3D). This would be in agreement with the reduction in ADAMTS13 IgG antibody levels and time to remission. Normalization of B-cell numbers occurred in 75% of patients, with levels above the normal range within 12 months (7.76%; range, 0.46%-32.5%). However, this was not associated with further relapse. Serum immunoglobulin levels (IgG and IgA) remained within the normal range; a reduction below the normal range was seen in IgM in some patients, but did not translate to an increase in infections.
(A) Effect of treatment on median ADAMTS13 activity and IgG antibody levels in box-whisper format from admission to 1 year of follow-up in the rituximab group. (B) Median ADAMTS13 activity in the trial patients from admission to 1 year of follow-up. (C) Median anti-ADAMTS13 IgG levels in trial patients from admission to 1 year of follow-up. (D) Effect of rituximab on CD19 levels (normal range, 5%-15%) before each rituximab infusion and at follow-up to 1 year. (E) Median ADAMTS13 activity and IgG antibody levels on admission, in remission, and on relapse in the historical group.
(A) Effect of treatment on median ADAMTS13 activity and IgG antibody levels in box-whisper format from admission to 1 year of follow-up in the rituximab group. (B) Median ADAMTS13 activity in the trial patients from admission to 1 year of follow-up. (C) Median anti-ADAMTS13 IgG levels in trial patients from admission to 1 year of follow-up. (D) Effect of rituximab on CD19 levels (normal range, 5%-15%) before each rituximab infusion and at follow-up to 1 year. (E) Median ADAMTS13 activity and IgG antibody levels on admission, in remission, and on relapse in the historical group.
Relapse
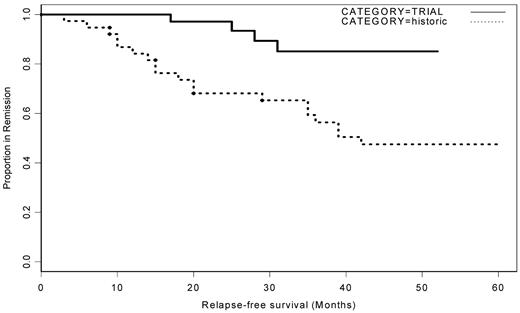
In the rituximab group, there were 4 relapses in the 37 surviving patients (Figure 4). No relapses occurred during the 12 months after initial presentation. The median time to relapse was 27 months (17, 25, 28, and 31 months). At that time, ADAMTS13 activity had decreased to < 5% in 3 patients; in the fourth, ADAMTS13 activity had remained < 5% after initial presentation and treatment. One patient died in relapse, and the remaining 3 patients were successfully retreated with rituximab. In the historical controls, 21 of 38 patients had an acute TTP relapse at a median of 18 months (range, 3-60 months) after admission, with 6 patients relapsing within the first year of presentation. The reduction in relapse from 57% to 10% was highly significant (P = .0011; Figure 2). In the historical group, 24 patients received only PEX with or without steroids upon acute admission. Fifty percent (12 of 24) of this group had a further relapse and one patient died. Nine of the 15 patients (60%) who required other immunosuppressive therapy had further relapses, and 2 of the 15 died.
Acute TTP relapse in trial patients receiving rituximab compared with historic controls. Kaplan-Meier curve of relapse-free period of historical controls compared with patients who received rituximab. Over a 49-month period, more than half of the historical control patients had a further acute TTP episode. This was significantly reduced in the rituximab group.
Acute TTP relapse in trial patients receiving rituximab compared with historic controls. Kaplan-Meier curve of relapse-free period of historical controls compared with patients who received rituximab. Over a 49-month period, more than half of the historical control patients had a further acute TTP episode. This was significantly reduced in the rituximab group.
ADAMTS13
The effect of rituximab on ADAMTS13 and anti-ADAMTS13 IgG in the trial group is shown in Figure 3A. The median ADAMTS13 activity was < 5% (range, 0%-32%) at presentation, with the median positive IgG antibodies to ADAMTS13 at 40% (range, 6%-162%). Before the second rituximab infusion, there was an increase in ADAMTS13 activity, with the median levels at 15%. Overall, the reduction in anti-ADAMTS13 IgG levels was associated with an increase ADAMTS13 activity, with a correlation coefficient of −0.72 (Pearson correlation), demonstrating that the decrease in anti-ADAMTS13 IgG was associated with an increase in ADAMTS13 activity. Of the trial patients who relapsed, 2 were of Afro-Caribbean ethnicity, and 1 of them achieved clinical remission after the first presentation. However, ADAMTS13 activity remained < 5%, although there was a reduction of anti-ADAMTS13 antibody level. Another patient only achieved an increase in ADAMTS13 activity to approximately 30% after the initial TTP episode and 4 weekly rituximab treatments. After retreatment with rituximab on relapse 31 months later, the patient's ADAMTS13 level returned to the normal range.
In the historical control group, the median ADAMTS13 activity on admission was < 5% with an anti-ADAMTS13 IgG of 78% (range, 8%-140%). In remission, the overall median ADAMTS13 activity was below the normal range at 12% (range, ≤ 129%), with detectable antibody (median 20%; range, 5%-69%). On relapse, the median ADAMTS13 activity was < 5% with anti-ADAMTS13 antibody levels of 29% (Figure 3E).
Adverse effects
During admission and up to 1 year at follow-up, no increase in infections was documented. Mild joint pain mainly affecting the knees and responding to simple analgesics during follow-up occurred in a minority of patients. Chest pain temporally related to the rituximab infusion was noted. In one patient there was a significant increase in troponin T and another patient had chest pain during the infusion. Because this primarily occurred in association with the first infusion, it was difficult to differentiate if it was caused by rituximab itself or by the underlying disease (Table 3).
Adverse events occurring during rituximab therapy and throughout the 1-year follow-up period
| Event . | n . |
|---|---|
| Total deaths | 3 |
| Deaths caused by cerebral infarction and heart involvement | 1 |
| Deaths due to cardiac TTP | 2 |
| Chest pain | |
| Chest pain during IMP infusion | 1 |
| Chest pain following IMP infusion | 5 (1 associated with troponin T > 0.05) |
| Chest pain not related to IMP | 2 |
| Infections | |
| Infections following rituximab infusion (up to 1-y follow-up) | 26 |
| Viral infections | 10 |
| Bacterial infections | 1 (Clostridium difficile) |
| Skin infections | 4 (2 Staphylococcus aureus; 1 fungal) |
| Cellulitis | 2 |
| Urinary tract infections | 6 (3 E coli, twice in same patient; 1 Enterococcus) |
| Infections due to infusion lines | 3 |
| Infections prior to rituximab infusion/unrelated to rituximab | 10 (1 E coli; S aureus) |
| Neurologic | |
| TIA | 4 (3 occurring sequentially in same patient) |
| Numbness in limb | 4 (2 in same patient) |
| Depression after discharge | 3 |
| Sensory/motor abnormalities not related to IMP | 5 |
| Headaches | 4 |
| Hematologic | |
| Reduced neutrophil count (< 1.5 × 109/L) | 3 (transitory, incidental, no infections) |
| Reduced platelet count | 1 |
| Vascular | |
| Deep vein thrombosis | 1 |
| Increased blood pressure | 2 |
| Hypotensive | 2 |
| Other vascular | 2 |
| Reproductive | |
| Miscarriage unrelated to rituximab | 1 |
| Miscarriage (partner's) | 1 |
| Stillbirth (partner's) | 1 |
| Other events | |
| Joint pain possibly related to rituximab | 5 |
| Skin rash (in remission) possibly related to rituximab | 3 |
| Hair loss/thinning possibly related to rituximab | 2 |
| Temperature 38°C | 3 |
| Event . | n . |
|---|---|
| Total deaths | 3 |
| Deaths caused by cerebral infarction and heart involvement | 1 |
| Deaths due to cardiac TTP | 2 |
| Chest pain | |
| Chest pain during IMP infusion | 1 |
| Chest pain following IMP infusion | 5 (1 associated with troponin T > 0.05) |
| Chest pain not related to IMP | 2 |
| Infections | |
| Infections following rituximab infusion (up to 1-y follow-up) | 26 |
| Viral infections | 10 |
| Bacterial infections | 1 (Clostridium difficile) |
| Skin infections | 4 (2 Staphylococcus aureus; 1 fungal) |
| Cellulitis | 2 |
| Urinary tract infections | 6 (3 E coli, twice in same patient; 1 Enterococcus) |
| Infections due to infusion lines | 3 |
| Infections prior to rituximab infusion/unrelated to rituximab | 10 (1 E coli; S aureus) |
| Neurologic | |
| TIA | 4 (3 occurring sequentially in same patient) |
| Numbness in limb | 4 (2 in same patient) |
| Depression after discharge | 3 |
| Sensory/motor abnormalities not related to IMP | 5 |
| Headaches | 4 |
| Hematologic | |
| Reduced neutrophil count (< 1.5 × 109/L) | 3 (transitory, incidental, no infections) |
| Reduced platelet count | 1 |
| Vascular | |
| Deep vein thrombosis | 1 |
| Increased blood pressure | 2 |
| Hypotensive | 2 |
| Other vascular | 2 |
| Reproductive | |
| Miscarriage unrelated to rituximab | 1 |
| Miscarriage (partner's) | 1 |
| Stillbirth (partner's) | 1 |
| Other events | |
| Joint pain possibly related to rituximab | 5 |
| Skin rash (in remission) possibly related to rituximab | 3 |
| Hair loss/thinning possibly related to rituximab | 2 |
| Temperature 38°C | 3 |
TTP indicates thrombocytopenic purpura; IMP, investigational medicinal product; and TIA, transient ischaemic attack.
Discussion
The majority of acute acquired TTP presentations (> 70%) are now recognized as being autoimmune and antibody mediated, primarily IgG to ADAMTS13. These patients are more likely to present with end organ dysfunction, especially cardiac and neurologic symptoms, including coma.14 The efficacy of PEX treatment is well established,15 and patients with anti-ADAMTS13 antibodies require more PEX and therefore more plasma.3 However, there are limited data clearly demonstrating the utility of other immunosuppressive therapies in the management of TTP. Several small patient series describe various therapies, such as vincristine,16 cyclophosphamide, or splenectomy.17 Ciclosporin has been used with beneficial responses within 7-14 days of therapy; however, 14% of patients relapsed while on therapy and 33% relapsed within 3 months of stopping.18,19 There are no studies adequately powered to prove consistent clinical benefit.
Rituximab (MabThera; Roche Pharmaceuticals) is a monoclonal anti-CD20 antibody that specifically depletes B lymphocytes. Prospective studies and smaller case series have successfully and safely used rituximab, usually as a second-line therapy in patients with acute TTP who fail to respond to standard daily PEX and steroids or in relapsed acute idiopathic TTP patients who have previously demonstrated antibody to ADAMTS13 and electively choose to take rituximab to prevent further relapses.20-22 However, no study has demonstrated the safety or efficaciousness of rituximab given at the time diagnosis.
We present the results of a phase 2 trial using weekly rituximab, started within 3 days of admission. The trial group was compared with a historical control group that was matched as far as possible for age, sex, and ethnic group. There were a greater number of Afro-Caribbean patients in the trial group than would be expected from the local population. A high proportion of trial patients were intubated and ventilated on admission (15%) and admitted to the ICU, but the frequency of all other presenting clinical features were consistent between the groups. The exception was the measurement of troponin levels, which was not undertaken in the historical group.
Our current treatment protocol involves intensive PEX on admission, especially in patients with neurologic and cardiac involvement and those who are intubated, who typically receive twice-daily PEX. Refractory/recurrent relapsing TTP patients admitted into the ICU setting have been shown to require more PEX treatments to achieve remission.8 In antibody-mediated disease, there is evidence of increased relapse rates,23,24 a requirement for more therapy to remission,8 and an association with autoimmune disease,25 especially in nonwhite patients. In our study cohort, the median number of PEX treatments was 16.5 compared with 18 in the historical controls. When the study parameters were divided into ethnic groups, white patients required significantly fewer PEX treatments than nonwhite patients receiving rituximab (mean difference, 7 days of PEX).
Because PEX intensity and schedules may change over time, in addition to using PEX as an end point, we also looked at the number of inpatient days. There was no overall difference in the length of stay between trial patients and historical controls; however, when patients who were admitted to the ICU were removed from the analysis, there was a statistically significant reduction in length of stay in those who received rituximab compared with the historical controls. When the different ethnic groups were analyzed, there was a significant reduction in length of stay for white patients who received rituximab compared with historical controls that was not seen in the other ethnic groups. The response in this cohort suggests a benefit of rituximab therapy given at acute presentation, even in patients presenting with the severest disease phenotype.
Our cohort included a greater number of Afro-Caribbean patients in the rituximab group than expected. Afro-Caribbean patients required more rituximab, based on their time to remission and the ADAMTS13 activity and anti-ADAMTS13 IgG levels. Nonwhite patients overall required significantly more PEX treatments until remission compared with white patients.
Relapse in TTP remains problematic and is reported to occur in 20%-50% of patients.26-30 In the South East England TTP registry, > 50% of the recorded episodes were relapses.14 Relapse in TTP has been shown to be associated with the persistence of anti-ADAMTS13 antibodies and persistent low ADAMTS13 activity in remission, as demonstrated in our historical control group. We have demonstrated a significant reduction in the relapse risk in the trial patients receiving rituximab compared with historical controls in this cohort and data in the literature. In the trial group, 10% of patients relapsed, compared with > 50% in the historical control group. Of the 14 patients who did not fit the inclusion criteria and received rituximab off-trial, only 2 subsequently relapsed in the 36 months of follow-up; both of these patients were adolescents. Approximately one-third of the historic controls in our cohort received treatment with one or more immunosuppressive treatments in conjunction with PEX and steroids. The criteria used as indications for the use of additional therapies were failure to achieve a normal platelet count at 1 week and/or deterioration in clinical symptoms.2 Fifty percent of these patients subsequently relapsed and 2 patients died. In those historic control patients treated with PEX with or without steroids (and no additional immunosuppression), 50% also relapsed. This suggests that PEX treatment and steroids alone, and often the use of other immunosuppressive therapies as well, are not sufficient to switch off the autoimmune process and prevent further relapse. However, with anti-CD20 therapy, remission was achieved and there was a sustained benefit within all ethnic groups.
CD19, a marker of B lymphocytes, was elevated at admission in the rituximab group (not measured in the historical controls). CD19 levels began to decrease by the second rituximab infusion, and fell below the normal range, between the second and third rituximab infusions (at days 8 and 15), around the time of platelet normalization. An increase in CD19 to normal levels during follow-up was not associated with clinical relapse.
The use of rituximab in TTP patients was associated with a decrease in anti-ADAMTS13 IgG and a rise in ADAMTS13 activity. In this group, the median ADAMTS13 increased significantly, from < 5% on admission to 45% at 3 months, with a decrease in anti-ADAMTS13 IgG. In contrast, there were a variety of immunosuppressive therapies used in the historical controls, none of which appeared to reduce relapse and, where available, showed no effect on ADAMTS13 activity/anti-IgG antibody levels. Forty-seven percent of the historical controls had ADAMTS13 activity < 10% in remission with detectable antibody. Of the 4 patients from the trial group who relapsed, ADAMTS13 activity had decreased < 5% in 3 patients; in the fourth, ADAMTS13 activity had remained < 5% after initial presentation and treatment. All of the other remaining patients had normal ADAMTS13 activity in remission well after the 1-year follow-up period. All were retreated with rituximab, 3 successfully and 1 who died (as documented in the mortality data for the trial).
Whereas the long-term effects of rituximab remain unknown, the safety profile compared with other immunosuppressive therapies, including steroids, cyclosporin, or cyclophosphamide, appears considerably improved. We have not seen any complications within the trial that have resulted in a compromise in safety and no increase in infections was demonstrated. There were some cases of chest pain temporally related to rituximab infusion; however, it is often difficult to differentiate drug effect from the underlying disease (although in 1 patient, there was chest pain during the infusion). It would be expected that the reduction in B-cell numbers could result in an increased incidence of infections. Despite the median CD19 levels being below the normal range for at least 12 months, there was no obvious increase in infection throughout this period. We have previously demonstrated clearance of rituximab during PEX and failure to attain detectable trough levels before the next infusion,31 suggesting that the frequency of infusions in acute TTP may need to be reviewed.
In conclusion, we have demonstrated that the use of weekly rituximab in conjunction with standard therapy (PEX and steroids) was safe, efficacious, and well tolerated. There was a significant reduction in the number of inpatient days in those not admitted to the ICU. In addition, there was a (nonsignificant) reduction in the mean number of PEX treatments until remission when analyzing all patients together, but a significant reduction in white patients receiving rituximab compared with nonwhite. The relapse rate was also significantly reduced compared with the control group and with data published in the literature. This relapse rate was associated with a reduction in anti-ADAMTS13 IgG and an increase in ADAMTS13 activity. The reduction in relapse rate suggests that anti-CD20 therapy switches off anti-ADAMTS13 antibody production, allowing for sustained remission. Maximizing therapy for patients with clinical signs of end organ dysfunction, especially cardiac and neurologic features, with intensive PEX treatment, steroids, and early use of rituximab improves time to remission acutely and prevents recurrent relapses.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Fritz Scheiflinger (Baxter, Austria) for providing rADAMTS13 for the antibody assay.
Roche Pharmaceuticals (United Kingdom) supplied rituximab (MabThera) for all patients entering the trial. V.M. is funded by an unrestricted educational grant from Baxter (United Kingdom).
Authorship
Contribution: M.S., J.C., and B.J.H. designed the study; M.S., J.C., B.J.H., V.M., and H.C. performed the study; and all authors analyzed the data and wrote the manuscript. All authors have access to the trial data and have been involved in the analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Marie Scully, Department of Haematology, 60 Whitfield Street, London W1T 4EU, United Kingdom; e-mail: m.scully@ucl.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal