Abstract
Acute graft-versus-host disease (GVHD) is a major complication of allogeneic stem cell transplantation (SCT) and can be readily controlled by systemic high-dose steroids in many patients. However, patients whose GVHD is refractory to this therapy have a poor prognosis. Refractory patients have ongoing end-organ damage despite effective immunosuppression with second-line regimens, suggesting pathomechanisms independent from the initiating T-cell attack. To explore whether endothelial damage might contribute to GVHD refractoriness and to study the role of angiopoietin-2 (ANG2) in this process, we have compared kinetics of T-cell activation markers and markers of endothelial dysfunction in the serum of patients with sensitive (n = 23) and refractory GVHD (n = 25). Longitudinal measurements of soluble FAS ligand along with other immune markers demonstrate that refractory patients are not exposed to an overwhelming or unresponsive T-cell attack. However, in contrast to sensitive GVHD, refractory GVHD was associated with rising thrombomodulin levels and high ANG2/ vascular endothelial-derived growth factor ratios. Patients with refractory GVHD showed significantly increased ANG2 levels already before SCT. These results suggest that endothelial cell vulnerability and dysfunction, rather than refractory T-cell activity, drives treatment refractoriness of GVHD and opens new avenues for prediction and control of this devastating condition.
Introduction
Acute graft-versus-host disease (GVHD) occurs in a large proportion of patients who have undergone allogeneic stem cell transplantation (SCT) and is a major contributor to mortality and morbidity associated with this procedure. Although GVHD can be readily controlled by escalation of systemic immunosuppression (usually high-dose steroids) in up to 70% of patients,1,2 it is resistant to first-line therapy in the remainder. Salvage treatment with alternative immunosuppressive drugs or anti-inflammatory agents can induce responses in a variable fraction of steroid-refractory patients. However, the overall outlook of steroid-refractory GVHD is generally poor with only few long-term survivors.2-5
The pathophysiology of steroid-refractory GVHD is poorly understood. GVHD is triggered by alloreactive cytotoxic T-lymphocytes that attack the recipient's organs.6 GVHD-targeted immunosuppressive salvage therapy efficiently eradicates T cells7 but fails to stop organ damage in refractory patients, as ongoing epithelial apoptosis can be measured by persistently increased serum cytokeratin-18 fragments (CK18F).8 These findings suggest that additional mechanisms might be relevant in advanced stages of GVHD, which perpetuate damage of target organs independent from the initial T-cell trigger. In this regard, antecedent reports have drawn attention to a potential role of the vascular endothelium in mediating end-organ damage in GVHD.9-11 In particular, increases of the endothelial activation markers von Willebrand factor12 and soluble thrombomodulin13-15 were found to be associated with the activity of acute GVHD.
Angiopoietin-2 (ANG2) is the TIE2-binding antagonist of angiopoietin-1 that controls vessel quiescence in adults.16 ANG2 strongly potentiates the effect of tumor necrosis factor-α on the induction of inflammatory gene transcription, such as ICAM-1 and VCAM-1 in endothelial cells, suggesting a permissive role for the activities of proinflammatory cytokines.17 High serum ANG2 levels were correlated with severity and mortality of sepsis18 as well as with vascular inflammation in autoimmune diseases, such as systemic lupus erythematodes.19 Moreover, specifically in the context of reduced vascular endothelial-derived growth factor (VEGF) levels (ie, a high ANG2/VEGF ratio), ANG2 mediates endothelial cell death and vessel regression.20,21 Higher VEGF levels have been associated with reduced severity of GVHD.22
Based on these preliminary findings, we hypothesized that endothelial damage might contribute to the development of steroid refractoriness of GVHD by perpetuating inflammatory end-organ destruction despite effective control of T-cell activity, and that ANG2 could play a role in the pathogenesis of the endothelial dysfunction underlying steroid-refractory GVHD. To dissect the individual contributions of T-cell activity and microangiopathy to apoptosis of end-organ target cells in steroid-refractory GVHD, we have compared the kinetics of ANG2 and soluble thrombomodulin (sTM) as markers of vascular endothelial homeostasis along with soluble FAS ligand (sFASL) and other T-cell activation markers between patients with refractory GVHD and those with sensitive GVHD. Our results indicate that sensitive and refractory patients have similar T-cell activation patterns. In contrast, only patients with steroid-refractory GVHD display serologic signs of escalating endothelial damage. In particular, ANG2 levels were found to be significantly higher in patients with refractory GVHD than in those with sensitive GVHD. The fact that increased ANG2 levels in patients with refractory GVHD were observed already before transplantation suggests that ANG2 might not only be a key player in the pathogenesis of steroid refractoriness but may also help to assess endothelial vulnerability that may result in a high frequency of refractory GVHD.
Methods
Patient eligibility
All patients eligible for this study had undergone SCT at our institution between January 2003 and June 2009 and fulfilled the following criteria: (1) one or more clinically and histopathologically proven (intestinal tract) or clinically and serologically proven (liver) episode of acute or chronic GVHD; and (2) availability of longitudinal serum samples for one year after transplantation or at least for a period flanking the GVHD episode for about one month or until death because of GVHD. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki and the local ethics committee of Heidelberg University Hospital had approved sample collection for retrospective analyses. Eighteen of these patients had been included in a previous study analyzing CK18F as marker for GVHD activity.8
Steroid resistance and GVHD grading
GVHD was clinically graded using standard criteria.23 Acute and chronic GVHD were subgrouped into categories according to the National Institutes of Health consensus criteria.24 Steroid-refractory GVHD was defined as disease clinically not responding to standard steroid therapy (2 × 1 mg/kg). Second-line salvage for refractory GVHD was pentostatin in all cases.5 Further salvage attempts were performed individually and composed of mesenchymal stem cells, tacrolimus, basiliximab, rituximab, and infliximab.
Serum samples and ELISAs
Serum samples were collected before SCT and thereafter in weekly or second weekly intervals for a maximum of 1 year. After blood collection, serum was immediately obtained by centrifugation, transferred into cryotubes, and stored at −80°C until further processing. Serum levels of CK18F were measured using the M30-Apoptosense ELISA Kit (Peviva) according to the manufacturer's instructions as reported previously.8 sFASL and sTM ELISA Kits were purchased from Diaclone. The absorbance at 450 nm was finally determined using a Sunrise absorbance reader, and the absorbance data were analyzed using Magellan software (both from Tecan).
Multiplex analyses
Concentrations of ANG2 and VEGF were quantified in patient sera at different time points by the multiplex protein array technology (Luminex) according to the manufacturer's instructions for protein multiplexing (Bio-Rad). Concentrations of the chemokines Fractalkine (CX3CL1), ENA-78 (CXCL5) and Eotaxin-3 (CCL26, MIP-4a) as well as TRAIL levels were determined by the by the bead-based multiplex protein array technology (Luminex), according to the manufacturer's instructions for protein multiplexing (Panomics Affymetrix). In brief, a 2-laser array reader simultaneously quantifies all proteins of interest with high sensitivity and high reproducibility. Human patient sera were diluted 1:2 with sample buffer; and for each parameter in each sample, at least 50 beads were counted, giving rise to 50 individual data points as median fluorescence intensity. On the basis of the standard curves for each parameter, concentrations for each sample were calculated by the Bio-Plex Manager Version 4.1.1 software on the basis of the 5-parameter logistic plot regression formula. The detection sensitivity of all analyses was between 2 pg/mL and 40 ng/mL.
Statistical analysis
Serum parameters (CK18F, sFASL, sTM, ANG2, CX3CL1, CXCL5, CCL26, and TRAIL) were measured longitudinally and compared, if appropriate, at the following landmarks using either the signed-rank test of Wilcoxon for comparisons of 2 time points or the Friedman test for more time points: before SCT (0), at the time of escalating immunosuppression (1), and late after escalation of immunosuppressive treatment (2; “follow-up”: means of all values measured between days 20 and 90). Differences of serum parameter levels (sFASL, sTM) between steroid-refractory and sensitive patients were calculated by the signed-rank test of Mann-Whitney. Categorical data of patient characteristics were compared using the 2-tailed Fisher exact test or the χ2 test.
A logistic regression model was used to predict therapy response (steroid-refractory vs -sensitive GVHD) based on categorized ANG2 levels (cut-off, 750 pg/mL) and categorized thrombomodulin levels (cut-off, 8 pg/mL) measured before SCT. Cut-off levels for categorization were empirically chosen based on median levels of ANG2 and thrombomodulin.
Survival data were analyzed as of November 2010. Overall survival was calculated as the time from allo-SCT to death of any cause. Nonrelapse mortality (NRM) was calculated as the time from allogeneic SCT to the endpoint death in the absence of relapse considering recurrence of the underlying malignancy as competing risk. Baseline serum levels of sTM (cut-off, 8 ng/mL) and ANG2 (750 pg/mL), which were determined shortly before transplantation, were analyzed in a univariate Cox regression of cause-specific hazards to evaluate their impact on NRM considering relapse as a competing risk.
A time-dependent Cox regression analysis of cause-specific hazards was performed to evaluate the prognostic impact of therapy response (steroid-refractory vs -sensitive GVHD) and categorized thrombomodulin levels (cut-off, 8pg/mL) measured before transplantation, at onset of GVHD, and during follow-up of the GVHD episode on NRM.
A second time-dependent Cox regression of cause-specific hazards was performed with the intention to analyze the prognostic impact of time-dependent categorized ANG2/VEGF ratios, (cut-off, 10), categorized thrombomodulin levels (cut-off, 8 pg/mL), and categorized sFASL levels (cut-off, 50 pg/mL) on NRM, including donor (related donor [RD] vs matched unrelated donor [MUD] vs mismatched unrelated donor [MMUD]), conditioning (reduced intensity conditioning [RIC] vs myeloablative conditioning [MAC]), and disease-specific score as defined by Gratwohl et al25 (0 vs 1 vs 2) as additional baseline covariates in the model. Serum parameters (sFASL, ANG2/VEGF ratio) were measured before transplantation, at onset of GVHD, and during follow-up of the GVHD episode. Calculations were done using the statistical software environment R Version 2.12.2, together with the R package “rms” Version 3.3-0 or SPSS Version 16.0, software. All statistical tests were 2-sided, and results with P values < .05 were considered to be statistically significant.
Results
Clinical patterns and outcome of GVHD
Forty-eight patients fulfilled the inclusion criteria for this study. Patient characteristics are summarized in Table 1. Twenty-three patients had sensitive and 25 patients had refractory GVHD. The median time interval from SCT to GVHD was 131 days (range, 20-213 days) in sensitive and 57 days (12-200 days) in refractory patients (P = .14). A total of 37 patients were affected by gastrointestinal GVHD with or without hepatic involvement (60% refractory), whereas 12 had hepatic GVHD only (17% refractory). Chronic courses of GVHD were observed in 13 patients (23% refractory, P = .114). Acute GVHD grade at disease onset (escalation) was 2 in the sensitive group and 2 in the refractory group (medians, P = .347), whereas maximum grade acute GVHD was 3 or 4 in all refractory patients. Sensitive patients had a higher incidence of overlap syndromes (Table 1). Steroid-refractory disease was associated with significantly higher NRM (P < .0001; hazard ratio [HR] = 27.8; 95% confidence interval [CI], 4.2-185.6), translating into a significantly worse overall survival of patients with refractory GVHD (P = .0003; HR = 13.9; 95% CI, 3.3-58.7; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Patient characteristics
| . | Steroid-resistant GVHD . | P . | |
|---|---|---|---|
| No (n = 23) . | Yes (n = 25) . | ||
| Median age at treatment, y (range) | 45 (22–56) | 48 (24–62) | .268* |
| Sex of recipient (female/male) | 11/12 | 11/14 | 1.000† |
| Donor | |||
| RD | 14 | 10 | .459† |
| MUD | 5 | 4 | .729† |
| MMUD | 4 | 11 | .232† |
| Sex of donor (female/male) | 5/18 | 12/13 | .256† |
| Sex mismatch R/D | 10 | 11 | 1.000† |
| Disease | |||
| AML, MDS | 7 | 12 | .586† |
| CML | 2 | 1 | .609† |
| ALL | 3 | 3 | 1.000† |
| Lymphoma, CLL | 5 | 4 | .729† |
| MM, amyloidosis | 6 | 5 | .748† |
| Disease score | |||
| 0 | 8 | 13 | .599† |
| 1 | 13 | 6 | .176† |
| 2 | 2 | 6 | .276† |
| ATG | 7 | 13 | .424† |
| Stem cell source (BM/PBSCs) | 1/22 | 1/24 | 1.000† |
| Conditioning | |||
| RIC | 13 | 16 | .817† |
| MAC | 10 | 9 | .790† |
| GVHD site | |||
| Gut only | 13 | 15 | 1.000† |
| Liver only | 10 | 2 | .048† |
| Gut and liver | 0 | 8 | .015† |
| Skin | 11 | 15 | .807† |
| GVHD type | |||
| Classic acute | 11 | 16 | .632† |
| Recurrent acute | 0 | 5 | .061† |
| Late-onset acute | 0 | 3 | .242† |
| Classic chronic | 4 | 0 | .111† |
| Overlap syndrome | 8 | 1 | .031† |
| GVHD prophylaxis | |||
| CsA | 22 | 23 | 1.000† |
| Tacrolimus | 1 | 1 | 1.000† |
| MMF (in combination) | 10 | 12 | 1.000† |
| MTX (in combination) | 9 | 9 | 1.000† |
| Cause of death | |||
| NRM | 1 | 17 | .001† |
| PD | 1 | 2 | 1.000† |
| . | Steroid-resistant GVHD . | P . | |
|---|---|---|---|
| No (n = 23) . | Yes (n = 25) . | ||
| Median age at treatment, y (range) | 45 (22–56) | 48 (24–62) | .268* |
| Sex of recipient (female/male) | 11/12 | 11/14 | 1.000† |
| Donor | |||
| RD | 14 | 10 | .459† |
| MUD | 5 | 4 | .729† |
| MMUD | 4 | 11 | .232† |
| Sex of donor (female/male) | 5/18 | 12/13 | .256† |
| Sex mismatch R/D | 10 | 11 | 1.000† |
| Disease | |||
| AML, MDS | 7 | 12 | .586† |
| CML | 2 | 1 | .609† |
| ALL | 3 | 3 | 1.000† |
| Lymphoma, CLL | 5 | 4 | .729† |
| MM, amyloidosis | 6 | 5 | .748† |
| Disease score | |||
| 0 | 8 | 13 | .599† |
| 1 | 13 | 6 | .176† |
| 2 | 2 | 6 | .276† |
| ATG | 7 | 13 | .424† |
| Stem cell source (BM/PBSCs) | 1/22 | 1/24 | 1.000† |
| Conditioning | |||
| RIC | 13 | 16 | .817† |
| MAC | 10 | 9 | .790† |
| GVHD site | |||
| Gut only | 13 | 15 | 1.000† |
| Liver only | 10 | 2 | .048† |
| Gut and liver | 0 | 8 | .015† |
| Skin | 11 | 15 | .807† |
| GVHD type | |||
| Classic acute | 11 | 16 | .632† |
| Recurrent acute | 0 | 5 | .061† |
| Late-onset acute | 0 | 3 | .242† |
| Classic chronic | 4 | 0 | .111† |
| Overlap syndrome | 8 | 1 | .031† |
| GVHD prophylaxis | |||
| CsA | 22 | 23 | 1.000† |
| Tacrolimus | 1 | 1 | 1.000† |
| MMF (in combination) | 10 | 12 | 1.000† |
| MTX (in combination) | 9 | 9 | 1.000† |
| Cause of death | |||
| NRM | 1 | 17 | .001† |
| PD | 1 | 2 | 1.000† |
R/D indicates recipient/donor; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; CML, chronic myeloid leukemia; ALL, acute lymphoid leukemia; CLL, chronic lymphocytic leukemia; MM, multiple myeloma; BM, bone marrow; PBSCs, peripheral blood stem cells; PD, progressive disease; CsA, cyclosporine A; MMF, mycofenolate mofetil; and MTX, methotrexate.
Mann-Whitney test.
Fisher exact test (2-tailed).
CK18F and T-cell activation markers
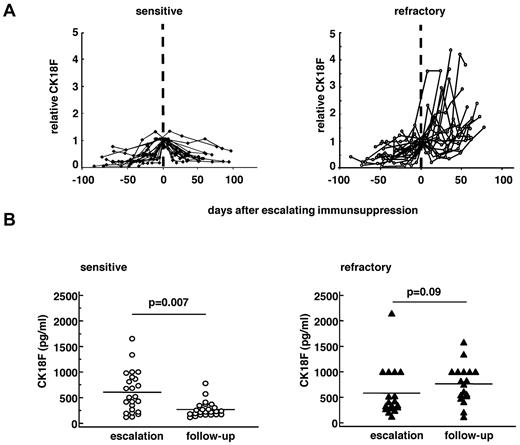
The kinetics of serum markers reflecting epithelial damage (CK18F) and T-cell activity were compared between patients with sensitive and refractory GVHD, respectively. In accordance with our previous report,8 serum CK18F levels rose before escalating immunosuppressive therapy and declined thereafter in patients with sensitive GVHD but not in patients with refractory GVHD (Figure 1). Absolute CK18F concentrations were similar in sensitive and refractory patients before SCT (sensitive, 164 U/mL; range, 81-320 U/mL; vs refractory, 173 U/mL; range, 20-313 U/mL, P = .85) and after GVHD onset before start of therapeutic immunosuppression (sensitive, 510 U/mL; range, 116-6680 U/mL; vs refractory, 345 U/mL; range, 120-2147 U/mL, P = .21). However, significantly higher CK18F levels were measured in steroid-refractory GVHD in the late period (follow-up), indicating the ongoing epithelial death of target organs (medians of all values per patient between day 20 and day 90, range): (sensitive, 224 U/mL; range, 113-780 U/mL; refractory, 607 U/mL; range, 115-1580 U/mL; P = .000 02).
Serum levels of CK18F in sensitive and refractory GVHD patients. Time course of individual serum CK18F (A) normalized to the day closest to escalation of immunosuppression (day 0) in 19 patients with therapy-sensitive (left panel) and 25 patients with refractory GVHD (right panel). (B) Absolute serum levels of CK18F in the individual sensitive (n = 19, left panel) and refractory (n = 25, right panel) GVHD patients at escalation of immunosuppression (“escalation”) and during the “follow-up” period (days 20-90 after escalating immunosuppression). Lines represent the medians. Wilcoxon signed-rank test was applied.
Serum levels of CK18F in sensitive and refractory GVHD patients. Time course of individual serum CK18F (A) normalized to the day closest to escalation of immunosuppression (day 0) in 19 patients with therapy-sensitive (left panel) and 25 patients with refractory GVHD (right panel). (B) Absolute serum levels of CK18F in the individual sensitive (n = 19, left panel) and refractory (n = 25, right panel) GVHD patients at escalation of immunosuppression (“escalation”) and during the “follow-up” period (days 20-90 after escalating immunosuppression). Lines represent the medians. Wilcoxon signed-rank test was applied.
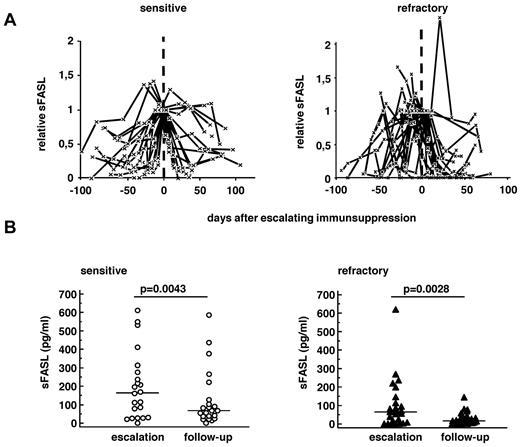
In contrast, sFASL levels rose before GVHD and decreased after escalating immunsuppressive therapy in both sensitive and refractory GVHD (Figure 2). Absolute serum levels at the time of escalating immunosuppressive therapy (escalation) and 20-90 days later (follow-up) are compared in Figure 2B. sFASL levels declined over time in both groups (P = .02 sensitive, P = .003 refractory) and were significantly lower in refractory than in sensitive patients in both periods of time (medians, escalation, sensitive 114 pg/mL, refractory 66 pg/mL, P = .05; follow-up, sensitive 67 pg/mL; refractory 9 pg/mL; P = .0001; Figure 2B).
Serum levels of sFASL in sensitive and refractory GVHD patients. Time course of individual sFASL levels (A) normalized to the day closest to escalation of immunosuppression (day 0) in 19 patients with therapy-sensitive (left panel) and 25 patients with refractory GVHD (right panel). (B) Absolute serum levels of sFASL in the individual sensitive (n = 19, left panel) and refractory (n = 25, right panel) GVHD patients at escalation of immunosuppression (“escalation”) and during the “follow-up” period (days 20-90 after escalating immunosuppression). Lines represent the medians. Wilcoxon signed-rank test was applied.
Serum levels of sFASL in sensitive and refractory GVHD patients. Time course of individual sFASL levels (A) normalized to the day closest to escalation of immunosuppression (day 0) in 19 patients with therapy-sensitive (left panel) and 25 patients with refractory GVHD (right panel). (B) Absolute serum levels of sFASL in the individual sensitive (n = 19, left panel) and refractory (n = 25, right panel) GVHD patients at escalation of immunosuppression (“escalation”) and during the “follow-up” period (days 20-90 after escalating immunosuppression). Lines represent the medians. Wilcoxon signed-rank test was applied.
Additional serologic T-cell activation markers (Fractalkine [CX3CL1], ENA-78 [CXCL5], Eotaxin-3 [CCL26] and TRAIL) were measured in 26 patients using multiplex analyses. In general, no differences were found between sensitive and refractory GVHD patients at escalation of immunosuppressive therapy, whereas late values were significantly higher in sensitive compared with refractory patients for ENA-78 and TRAIL with a similar trend in Eotaxin (supplemental Table 1). Although patient numbers were low, the results support the findings with sFASL indicating that refractory patients did not have stronger T-cell activation than sensitive patients. The lower serum levels of most T-cell markers in the late (days 20-90) period after the start of immunosuppression in refractory GVHD suggest that salvage immunosuppressive regimens were efficiently suppressing the T-cell arm of the immune response.
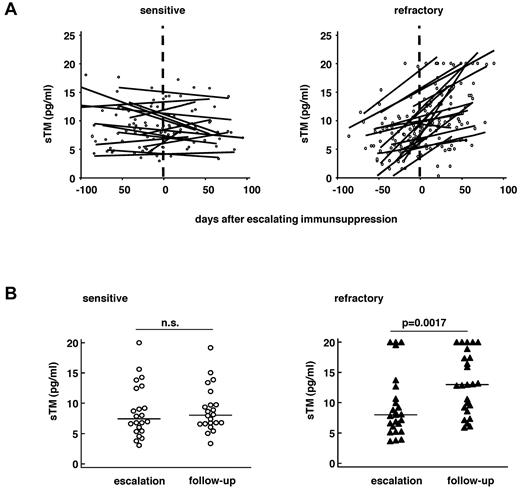
Kinetics of soluble thrombomodulin as marker of endothelial damage
As serum levels of sTM strongly fluctuated over time, sTM kinetics was visualized by linear regression lines. Results show an increase of sTM in steroid-refractory patients but not in sensitive patients after escalation of therapeutic immunosuppression (Figure 3). Before SCT and at escalation of immunosuppression, sTM levels did not differ in steroid-sensitive and -refractory patients (pre-SCT sensitive, 5.1 pg/mL; pre-SCT refractory, 5.8 pg/mL, P = .29; escalation sensitive, 7.6 pg/mL; escalation refractory, 8.3 pg/mL; P = .21; Figure 4A-B). However, sTM levels significantly increased in refractory patients in the follow-up period compared with escalation time (14.8 pg/mL vs 8.3 pg/mL; P = .0014), but not in sensitive patients (7.7 pg/mL vs 7.6 pg/mL; P = .71; Figure 3B), resulting in significantly higher sTM levels in refractory patients compared with sensitive patients at the follow-up time point (P = .0002; Figure 4C)
Serum thrombomodulin in sensitive and refractory GVHD patients. (A) Time course of individual sTM levels (pg/mL) in 22 patients with therapy-sensitive (left panel) and 25 patients with refractory GVHD (right panel). Shown are the individual data points and linear regression lines for each patient. (B) Absolute serum levels of sTM in individual sensitive (n = 23, left panel) and refractory (n = 25, right panel) GVHD patients at escalation of immunosuppression (“escalation”) and during the “follow-up” period (days 20-90 after escalating immunosuppression). Lines represent the medians. Wilcoxon signed-rank test was applied.
Serum thrombomodulin in sensitive and refractory GVHD patients. (A) Time course of individual sTM levels (pg/mL) in 22 patients with therapy-sensitive (left panel) and 25 patients with refractory GVHD (right panel). Shown are the individual data points and linear regression lines for each patient. (B) Absolute serum levels of sTM in individual sensitive (n = 23, left panel) and refractory (n = 25, right panel) GVHD patients at escalation of immunosuppression (“escalation”) and during the “follow-up” period (days 20-90 after escalating immunosuppression). Lines represent the medians. Wilcoxon signed-rank test was applied.
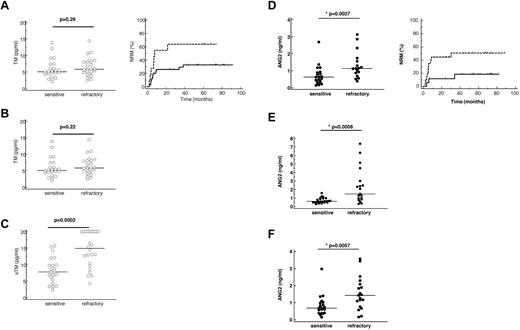
Serum thrombomodulin and ANG2 levels in sensitive and refractory GVHD patients correlate with NRM. Left panels: Absolute serum levels of sTM (A-C) and ANG2 (D-F) in 23 sensitive and 25 refractory patients before stem cell transplantation (A,D), at escalation of immunosuppression (B,E), and in the follow-up period (C,F). Lines represent the medians. Mann-Whitney test was applied. Right panels: (A-C) Univariate analyses of influence on NRM comparing patients with high sTM (> 8 pg/mL, dotted lines) and patients with low sTM levels (< 8 pg/mL, thick lines). (D-F) Univariate analyses of influence on NRM comparing patients with high ANG2 (> 750 pg/mL, dotted lines) and patients with low ANG2 levels (< 750 pg/mL, thick lines).
Serum thrombomodulin and ANG2 levels in sensitive and refractory GVHD patients correlate with NRM. Left panels: Absolute serum levels of sTM (A-C) and ANG2 (D-F) in 23 sensitive and 25 refractory patients before stem cell transplantation (A,D), at escalation of immunosuppression (B,E), and in the follow-up period (C,F). Lines represent the medians. Mann-Whitney test was applied. Right panels: (A-C) Univariate analyses of influence on NRM comparing patients with high sTM (> 8 pg/mL, dotted lines) and patients with low sTM levels (< 8 pg/mL, thick lines). (D-F) Univariate analyses of influence on NRM comparing patients with high ANG2 (> 750 pg/mL, dotted lines) and patients with low ANG2 levels (< 750 pg/mL, thick lines).
Interestingly, although no differences between sensitive and refractory patients were measured, serum levels of TM higher than 8 pg/mL measured before transplant were already predictive for NRM (Figure 4A right panel; P = .043; HR = 2.57; 95% CI, 1.0-6.6). In a time-dependent Cox regression model of cause-specific hazards, increasing TM levels during the time after transplantation > 8 pg/mL (P = .019; HR = 11.8; 95% CI, 1.5-9.4) and therapy response to steroids (P = .002; HR = 24.2; 95% CI, 3.1-188) were also associated with increased NRM mortality.
Kinetics of ANG2 levels and ANG2/VEGF ratio as markers of endothelial vulnerability
Loss of endothelial thrombomodulin coinciding with high serum levels of sTM represent ongoing endothelial damage. However, the reason why refractory patients have a more vulnerable endothelial cell system is not clear. We therefore measured ANG2 and VEGF levels as both markers have been associated with endothelial vulnerability.16-20
Serum levels of ANG2 showed no significant change over time in both cohorts but were significantly higher in refractory than in sensitive patients at all time points studied (pre-SCT, initiation of therapeutic immunosuppression, and follow-up; Figure 4D-F). Logistic regression analysis proved that ANG2 levels > 750 pg/mL before transplantation predict a steroid-refractory course of GVHD. Most interestingly, high ANG2 levels (> 750 pg/mL) measured before allo-SCT were also associated with worse NRM as determined by Cox regression analysis (Figure 4D right panel; P = .046; HR = 3.4; 95% CI, 1.0-12.6). This suggests that factors intrinsic to the endothelial cell system of the recipient may influence the outcome of allo-SCT.
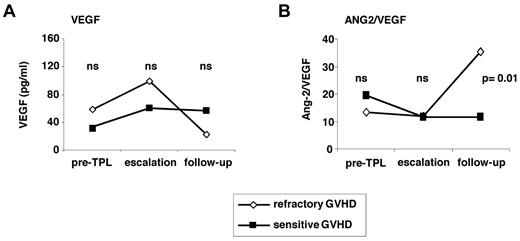
In contrast, the levels of VEGF as functional antagonist of ANG2 did not differ significantly between sensitive and refractory patients. However, in refractory patients, VEGF levels strongly decreased after escalation of immunosuppression (escalation, 111.7 pg/mL; range, 13.9[range]487.9 pg/mL; follow-up, 23.4 pg/mL; range, 1.2[range]215.3 pg/mL, P = .008; Figure 5A). This resulted in a significantly higher ANG2/VEGF ratio in refractory compared with sensitive patients at the follow-up time point (Figure 5B).
Serum VEGF levels and ANG2/VEGF ratios in sensitive and refractory GVHD patients. (A-B) Medians of serum VEGF levels (A) of 23 sensitive and 25 refractory GVHD patients and medians of the ANG2/VEGF ratios (B) of the same patients. ns indicates not significant. Mann-Whitney test was applied.
Serum VEGF levels and ANG2/VEGF ratios in sensitive and refractory GVHD patients. (A-B) Medians of serum VEGF levels (A) of 23 sensitive and 25 refractory GVHD patients and medians of the ANG2/VEGF ratios (B) of the same patients. ns indicates not significant. Mann-Whitney test was applied.
Multivariate prognostic factor analysis
To adjust the influence of endothelial and T-cell markers on NRM for potential confounders, a multivariate time-dependent Cox regression analysis of cause-specific hazards was performed using the covariates sFASL (< 50 pg/mL vs > 50 pg/mL), ANG2/VEGF (< 10 vs > 10), donor (RD vs MUD vs MMUD), conditioning (RIC vs MAC), and disease status score as defined by Gratwohl et al (0 vs 1 vs 2).25 Low sFASL levels (< 50 pg/mL) and high ANG2/VEGF ratios as well as the use of a MMUD significantly associated with higher NRM (Table 2). If sTM was included instead of ANG2/VEGF ratios in the regression model, similar significance levels were calculated (not shown).
Time-dependent multivariable cause-specific Cox regression analysis of influence on NRM
| Covariate . | P . | HR . | 95% CI . |
|---|---|---|---|
| Donor | |||
| RD vs MUD | .04 | 8.0 | 1.10-56.8 |
| RD vs MMUD | .0001 | 19.7 | 4.2-91.4 |
| Conditioning | |||
| RIC vs MAC | .15 | 0.31 | 0.1-1.5 |
| disease score | |||
| 0 vs 1 | .80 | 1.15 | 0.5-2.9 |
| sFASL (cut-off, 50 pg/mL) | .0001 | 0.05 | 0.01-0.18 |
| ANG2/VEGF (cut-off, 10) | .0004 | 17.5 | 3.5-87.1 |
| Covariate . | P . | HR . | 95% CI . |
|---|---|---|---|
| Donor | |||
| RD vs MUD | .04 | 8.0 | 1.10-56.8 |
| RD vs MMUD | .0001 | 19.7 | 4.2-91.4 |
| Conditioning | |||
| RIC vs MAC | .15 | 0.31 | 0.1-1.5 |
| disease score | |||
| 0 vs 1 | .80 | 1.15 | 0.5-2.9 |
| sFASL (cut-off, 50 pg/mL) | .0001 | 0.05 | 0.01-0.18 |
| ANG2/VEGF (cut-off, 10) | .0004 | 17.5 | 3.5-87.1 |
Covariates included: ANG2/VEGF (< 10 vs 10), FASL (< 50 pg/mL vs > 50 pg/mL), donor (RD vs MUD vs MMUD), conditioning (RIC vs MAC), and disease score (0 vs 1 vs 2). Serum parameters were measured before SCT, at escalation of immunosuppression, and in the follow-up period.
RR indicates relative risk.
Discussion
It is a challenge to understand why patients die of immune-mediated diseases despite extensive immunosuppressive therapy. For GVHD, the standard explanation would usually rely on quantitative differences, such as “weak” and “strong” immune responses resulting in lower- and higher-grade GVHD. Clearly, mortality of patients with grade 3 or 4 GVHD is significantly higher than for patients with grade 1 or 2 GVHD.4 However, the predictive power of maximal GVHD grades at disease onset is weak.4,26,27 As Leisenring et al26 and MacMillan et al4 have demonstrated, the prognosis of patients is far more affected by response or nonresponse within the following 4-7 weeks, suggesting that the category of therapy response (“sensitive-refractory”) might be more suitable to subclassify GVHD. Why do some patients not respond to therapy and progress into higher-grade GVHD?
Our study addresses this problem by retrospectively examining 2 groups of patients (refractory and sensitive) for whom longitudinally collected serum samples were available. Measuring sFASL levels in serum as a surrogate marker of cytotoxic T-cell activity, we found that sFASL levels were not higher in refractory than in sensitive patients. Furthermore, sFASL levels declined even more strongly in refractory patients after escalation of immunosuppressive therapy, suggesting that clinical nonresponse did not correlate with persistent uncontrolled T-cell activity. This is supported by our results of measuring the kinetics of other mediators of T-cell activation. However, the observed reduction of sFASL levels after immunosuppressive therapy has to be cautiously interpreted, as refractory patients received more severe immunosuppressive salvage regimens triggered by clinical nonresponse. Nevertheless, the rising CK18F levels clearly do not correlate with the time course of sFASL levels in steroid-refractory patients. This strongly suggests that different or additional mechanisms underlie the persistent apoptosis of epithelial organs.
Several lines of evidence point at endothelial damage as a potential underlying cause of GVHD refractoriness.9-11 Increased serum levels of soluble thrombomodulin are a well-acknowledged indicator of endothelial dysfunction and have been observed in the context of GVHD and other complications occurring during the early posttransplantation phase.13-15 Here we show, for the first time, that sTM increases indeed occur with GVHD onset but becomes significant only during longer follow-up after escalation of immunosuppression and are restricted to those patients whose GVHD is refractory. This is in keeping with the concept of a progressive endothelial damage/microangiopathy in the context of steroid-refractory GVHD.
How could endothelial cell damage be involved in the pathogenesis of GVHD? Endothelial cells are part of the recipient's antigen-presenting cell compartment and are able to express major histocompatibility complex class I, class II, costimulatory molecules such as CD80 and CD86, and cytokines such as IL-12p70.28 Although endothelial cells are usually not recognized by T cells, this situation may differ in the setting of local inflammation or endothelial stress because of chemotherapy or radiotherapy or cytomegalovirus infection.29,30 Even if not directly attacked, bystander endothelial cells might become exposed to proinflammatory cytokines, such as tumor necrosis factor-α, and respond with stress-associated changes. Loss of endothelial thrombomodulin expression is one feature of endothelial stress. Thrombomodulin is a protective molecule catalyzing protein C activation and inhibiting mitochondrial apoptosis of endothelial cells.31 A protective role of the endothelial thrombomodulin–activated protein C axis has been demonstrated in vivo linking endothelial cell dysfunction to epithelial apoptosis.31 We speculate that endothelial cell damage contributes to end-organ damage and delays repair mechanisms that are in charge to restore organ function. The high ANG2/VEGF ratios in the follow-up period (days 20-90) represent a condition of endothelial cell toxicity resulting in endothelial apoptosis in experimental models.20,21
Hypoxia and inadequate capillary blood flow are alternative potential pathomechanisms of how endothelial dysfunction might translate into epithelial apoptosis. On the other side, acute GVHD is usually associated with neoangiogenesis.32 However, the exact mechanism of how endothelial dysfunction translates into epithelial damage in refractory GVHD has yet to be elucidated.
One possible factor driving progressive microangiopathy associated with refractoriness of GVHD could be ANG2. ANG2 sensitizes endothelial cells to proinflammatory cytokines, such as TNF-α,17 suggesting that patients with high ANG2 levels could develop a more vigorous endothelial stress response to a local T-cell attack than patients with low ANG2 levels. This concept is supported by our observation that patients with refractory GVHD show high ANG2 levels at all time points and even before transplantation. It is further substantiated by the finding of strongly elevated ANG2/VEGF ratios after GVHD onset in refractory, but not in sensitive, patients because it has been demonstrated that ANG2 mediates endothelial cell death if VEGF is concomitantly blocked.20,21 Accordingly, high ANG2/VEGF ratios predicted for a significantly inferior survival of our patients even after multivariate adjusting for potential confounders, such as human leukocyte antigen match, disease status, and conditioning intensity. This is in line with observations in other diseases that are characterized by local or systemic cytokine release, such as sepsis and systemic lupus erythematosus, where ANG2 levels also correlate with mortality and severity.19 However, ANG2 levels alone overlapped considerably in patients with sensitive and refractory GVHD, implying that additional players mediating endothelial vulnerability are probably involved.
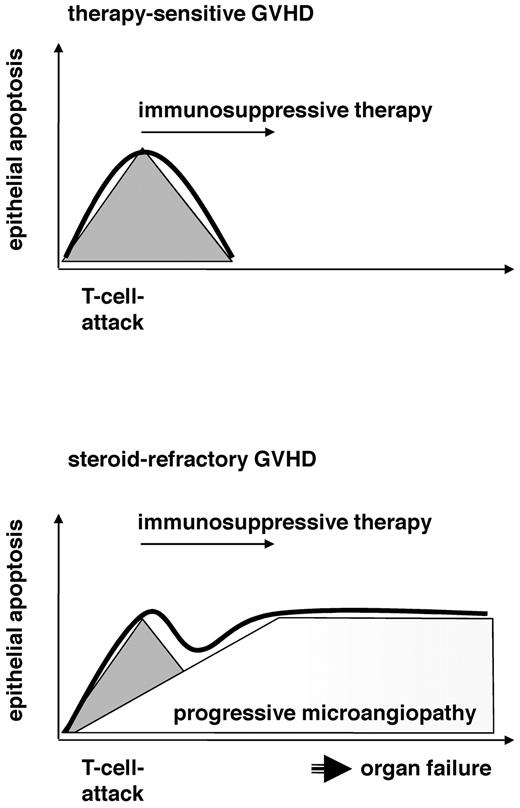
In conclusion, our results support the hypothesis that the pathogenesis of steroid-refractory GVHD involves progressive microangiopathy (Figure 6). T-cell attacks occur at the onset of both steroid-refractory and sensitive GVHD and similarly respond to immunosuppressive therapy, although refractory patients undoubtedly receive more severe immunosuppressive regimens. In contrast to sensitive GVHD, steroid-refractory disease is characterized by raising sTM serum levels and a sharp increase of the ANG2/VEGF-ratio, which is toxic to endothelial cells. It is probable that the cause of microangiopathy involves a predisposition/endothelial vulnerability because of the recipient's endothelial risk factors. We have demonstrated higher ANG2 serum levels in patients developing refractory GVHD; however, SNPs in genes relevant for endothelial homeostasis, types and amounts of previous therapies, cytomegalovirus, and comedication, etc could also play their roles.
Progressive microangiopathy distinguishes sensitive and refractory clinical courses of GVHD. The working hypothesis is as follows: Sensitive and refractory patients both have a T-cell attack (gray triangle) that responds to standard (sensitive) or salvage (refractory) immunosuppressive therapy. Epithelial apoptosis (thick line) is induced by T cells in sensitive patients and responds to immunosuppressive therapy after clearing the T-cell response (top panel). In contrast to sensitive patients, steroid-refractory patients have a vulnerable endothelial system that reacts to the initiating T-cell attack with progressive endothelial damage. This microangiopathy may cause organ failure and promote epithelial apoptosis, although the initiating T-cell attack was cleared (bottom panel).
Progressive microangiopathy distinguishes sensitive and refractory clinical courses of GVHD. The working hypothesis is as follows: Sensitive and refractory patients both have a T-cell attack (gray triangle) that responds to standard (sensitive) or salvage (refractory) immunosuppressive therapy. Epithelial apoptosis (thick line) is induced by T cells in sensitive patients and responds to immunosuppressive therapy after clearing the T-cell response (top panel). In contrast to sensitive patients, steroid-refractory patients have a vulnerable endothelial system that reacts to the initiating T-cell attack with progressive endothelial damage. This microangiopathy may cause organ failure and promote epithelial apoptosis, although the initiating T-cell attack was cleared (bottom panel).
What could be the clinical implication of these findings, apart from adding to our understanding of GVHD refractoriness? Given that markers of endothelial cell dysfunction can be prospectively validated in larger patient cohorts, they might be useful to improve algorithms used for estimating the individual risk of transplantation and defining allo-SCT indications. More importantly, they identify the endothelium as a promising target for prophylactic and therapeutic interventions aiming at prevention of fatal GVHD.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Helmholtz-Alliance on Immunotherapy of Cancer and the Wilhelm-Sander-Stiftung.
Authorship
Contribution: T.L. designed and performed research, analyzed data, and wrote the paper; S.D. performed research and statistical analysis of the data, discussed the data, and wrote the paper; C.F. performed Multiplex analyses, discussed the data, and provided ideas; A.B. performed statistical analysis of the data; M.C. performed research and provided ideas; M.H. performed research; F.N. gave advice for designing research, provided reagents, and discussed the data; B.I. and A.D.H. discussed the data and wrote the paper; U.H. performed research; and P.D. performed clinical research, discussed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Luft, University of Heidelberg, Department of Medicine V, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; e-mail: t.luft@dkfz.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal