Abstract
Unlike invasive aspergillosis, the prognosis and outcome of hematologic malignancy patients who develop invasive mucormycosis have not significantly improved over the past decade as a majority of patients who develop the infection still die 12 weeks after diagnosis. However, early recognition and treatment of invasive mucormycosis syndromes, as well as individualized approaches to treatment and secondary prophylaxis, could improve the odds of survival, even in the most persistently immunosuppressed patient receiving chemotherapy and/or of stem cell transplantation. Herein, we describe the subtle clinical and radiographic clues that should alert the hematologist to the possibility of mucormycosis, and aggressive and timely treatment approaches that may limit the spread of infection before it becomes fatal. Hematology patients with this opportunistic infection require integrated care across several disciplines and frequently highly individualized and complex sequence of decision-making. We also offer perspectives for the use of 2 antifungals, amphotericin B products and posaconazole, with activity against Mucorales. The availability of posaconazole in an oral formulation that can be administered safely for prolonged periods makes it an attractive agent for long-term primary and secondary prophylaxis. However, serum drug concentration monitoring may be required to minimize breakthrough infection or relapsing mucormycosis associated with inadequate blood concentrations.
Introduction
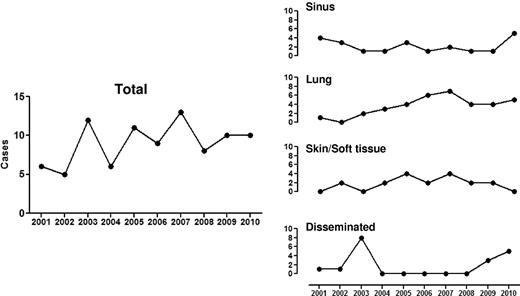
Mucormycosis is the unifying term used to describe infections caused by fungi belonging to the order Mucorales. Zygomycosis, an alternative term used to describe these life-threatening infections, has become less accurate based on a recent taxonomic reclassification (based on molecular identification) that abolished Zygomycetes as a class.1-4 Rhizopus, Mucor, and Rhizomucor species account for up to 75% of mucormycosis cases encountered in hematologic malignancy patients.5-7 Mucorales typically cause acute, aggressive, and frequently angioinvasive infections, especially in immunosuppressed hosts with hematologic malignancy and/or stem cell transplantation (SCT).8,9 This opportunistic, geographically broad mycosis was highlighted as a sporadic cause of death in leukemia patients in the 1950s10 until the mid 1990s where there has been an apparent increase in the documented cases in hematology units throughout the Westernized world (Figure 1).4-7,11 These cases are typically considered community-acquired, as nosocomial cases are less common in modern SCT and transplant units.12 However, the true incidence of mucormycosis is not known and probably underestimated because of difficulties in antemortem diagnosis and the low autopsy rates in contemporary patients who die in the setting of leukemia or SCT.13
Documented cases of mucormycosis (EORTC/MSG criteria) in Hematology and SCT services at M. D. Anderson Cancer, Houston, TX, from 2001 to 2010.
Documented cases of mucormycosis (EORTC/MSG criteria) in Hematology and SCT services at M. D. Anderson Cancer, Houston, TX, from 2001 to 2010.
The predisposing factors for mucormycosis in patients with hematologic cancer are similar to ones encountered in other opportunistic mold infections, such as aspergillosis, including profound and protracted neutropenia and monocytopenia, chronic high-dose corticosteroids, reactivation of opportunistic herpes viruses (especially cytomegalovirus), severe graft-versus-host disease and its treatment, active hematologic malignancy, and its associated functional neutropenia, as well as and high-risk SCT transplantation (Table 1).1-5,14 In addition, uncontrolled hyperglycemia, frank diabetes mellitus and/or diabetic ketoacidosis, and iron overload (associated with multiple transfusions of blood products for thalassemias or leukemic states) appear important contributing factors to increase mucormycosis risk (Table 1),2,5,14-18 although the relative contribution of each condition to mycormycosis risk is unknown. Finally, breakthrough mucormycosis has been increasingly observed in patients with leukemia and recipients of SCT receiving Aspergillus-active drugs, such as voriconazole or the echinocandins,5,19 as both agents that have no anti-Mucorales activity.20 Although the strongest association with breakthrough mucormycosis has been reported with voriconazole, other known and unknown risk factors may be more common in the higher-risk patient who received voriconazole prophylaxis,21 including (1) increased suspicion for mucormycosis, (2) prolonged survival after transplant to periods where other immunosuppressive risk factors that favor mucormycosis dominate (ie, corticosteroid-associated hyperglycemia, iron overload),15 and (3) increased environmental exposures in contemporary hematology patients who increasingly are treated in the outpatient basis.22 For example, a recent large prospective study comparing fluconazole and voriconazole prophylaxis in low- to moderate-risk allogeneic SCT recipients did not find an association between voriconazole prophylaxis and increased risk for breakthrough mucormycosis.23 As mucormycosis cases are typically seen in high-risk leukemia and allogeneic SCT recipients,5 it remains unclear what effects prolonged voriconazole exposure may have in patients to reduce the threshold of breakthrough mucormycosis. Given the multiple, interrelated risk factors for invasive fungal infections present in most patients with mucormycosis, it is often impossible to ascribe a single factor that increases the risk and or worsens the prognosis of this devastating infection.21
Predisposing risk factors for mucormycosis in patients with hematologic malignancies and/or stem cell transplantation
| Prolonged (> 3 wk) and severe (ANC < 200) neutropenia |
| Monocytopenia (< 100 mm3) |
| Prolonged (> 3 wk) high-dose systemic corticosteroids (eg, prednisone or equivalent of > 1 mg/kg/d) |
| Iron overload (assessed by high iron indices, high iron storage by MRI, or high iron staining in bone marrow biopsy) |
| High-risk SCT (eg, matched-unrelated donor SCT, haploidentical donor SCT, cord blood SCT, T cell-depleted SCT) |
| Severe GVHD and its treatment (especially corticosteroids) |
| Prolonged hyperglycemia (fasting serum glucose > 200 mg/dL), corticosteroid-associated hyperglycemia, diabetes mellitus |
| Colonization by mucormycetes or heavy environmental exposure? |
| Previous exposure to Aspergillus-active antifungal agents, especially voriconazole? |
| Relapsed leukemia |
| Prolonged (> 3 wk) and severe (ANC < 200) neutropenia |
| Monocytopenia (< 100 mm3) |
| Prolonged (> 3 wk) high-dose systemic corticosteroids (eg, prednisone or equivalent of > 1 mg/kg/d) |
| Iron overload (assessed by high iron indices, high iron storage by MRI, or high iron staining in bone marrow biopsy) |
| High-risk SCT (eg, matched-unrelated donor SCT, haploidentical donor SCT, cord blood SCT, T cell-depleted SCT) |
| Severe GVHD and its treatment (especially corticosteroids) |
| Prolonged hyperglycemia (fasting serum glucose > 200 mg/dL), corticosteroid-associated hyperglycemia, diabetes mellitus |
| Colonization by mucormycetes or heavy environmental exposure? |
| Previous exposure to Aspergillus-active antifungal agents, especially voriconazole? |
| Relapsed leukemia |
ANC indicates absolute neutrophil count.
Because documented mucormycosis is at least 5- to 10-fold less common than other molds, such as aspergillosis,5,13 many hematologists may not have accumulated a “critical mass” of experience with such cases to recognize early clinical signs that may be suggestive of invasive mucormycosis. Unfortunately, the aggressive nature of the infection often means that delayed diagnosis and erroneous treatment decisions often result in significantly higher patient morbidity and mortality.8 Furthermore, there are no controlled, randomized primary data in this complex area of clinical mycology or consensus treatment guidelines. In the following sections, we present our approaches to early diagnosis of management of mucormycosis in the hematology patient.
When should I suspect mucormycosis over aspergillosis?
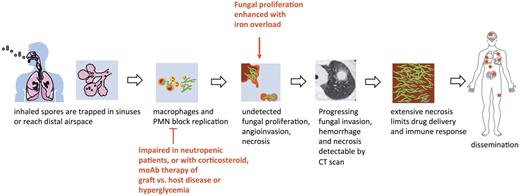
As the pathophysiology, mode of acquisition and underlying patient risk factors for mucormycosis are similar to aspergillosis (Figure 2), clinical distinction between the 2 entities is difficult. Both infections are acquired primarily through inhalation of spores, which are ubiquitous in the environment leading to sinopulmonary disease.1-4,14 Nevertheless, some scenarios and underlying risk factors and elements of the clinical and radiographic presentation should prompt a high index of suspicion for incipient mucormycosis5,19,24,25 (Table 2). Other clinical forms of mucormycosis, including primary cutaneous, gastrointestinal, rhino-orbital, or single-organ involvement are less common in hematology patients compared with other classic risk groups, such as the diabetic patients (rhino-orbital or rhinocerebral form), trauma victims, or premature neonates (primary cutaneous form).1-4 Mucormycosis is also more likely to initially present as a disseminated infection in severely immunosuppressed patients, even though the extent of dissemination is rarely appreciated antemortem.9
Factors favoring mucormycosis over aspergillosis
| Clues . | Comments . |
|---|---|
| Epidemiologic and host clues | |
| Institution with high background rates of mucormycosis5 | Unique geographic exposures vs institution-specific differences in immunosuppression and anti-infective practices |
| Iron overload16,17 | The most reliable method of diagnosis is unclear |
| Hyperglycemia with or without DM5 | Degree and duration are undefined |
| Prior voriconazole or echinocandin use5,18,24 | The magnitude and specificity of such association are debatable |
| Clinical, radiologic, and laboratory clues | |
| Community-acquired sinusitis5,24 | Pansinusitis or ethmoid involvement are important clinical clues of mucormycosis |
| Oral necrotic lesions in hard palate or nasal turbinates4 | |
| Chest wall cellulitis adjacent to a lung infarct4 | Mucormycosis can spread across tissue planes |
| Acute vascular event (eg, MI, GI bleeding)4 | Resulting from the acute hemorrhagic infarct caused by Mucorales |
| Multiple (n > 10) nodules in CT and pleural effusion24 | |
| Reverse halo sign in CXR or CT25 | Halo sign is as common in IPM as in IPA |
| Presumed (by CT findings) fungal pneumonia with adequate (eg, > 2 μg/mL) voriconazole levels19 | |
| Presumed (by CT findings) fungal pneumonia with repetitively negative GM and G-glucan serum levels24 |
| Clues . | Comments . |
|---|---|
| Epidemiologic and host clues | |
| Institution with high background rates of mucormycosis5 | Unique geographic exposures vs institution-specific differences in immunosuppression and anti-infective practices |
| Iron overload16,17 | The most reliable method of diagnosis is unclear |
| Hyperglycemia with or without DM5 | Degree and duration are undefined |
| Prior voriconazole or echinocandin use5,18,24 | The magnitude and specificity of such association are debatable |
| Clinical, radiologic, and laboratory clues | |
| Community-acquired sinusitis5,24 | Pansinusitis or ethmoid involvement are important clinical clues of mucormycosis |
| Oral necrotic lesions in hard palate or nasal turbinates4 | |
| Chest wall cellulitis adjacent to a lung infarct4 | Mucormycosis can spread across tissue planes |
| Acute vascular event (eg, MI, GI bleeding)4 | Resulting from the acute hemorrhagic infarct caused by Mucorales |
| Multiple (n > 10) nodules in CT and pleural effusion24 | |
| Reverse halo sign in CXR or CT25 | Halo sign is as common in IPM as in IPA |
| Presumed (by CT findings) fungal pneumonia with adequate (eg, > 2 μg/mL) voriconazole levels19 | |
| Presumed (by CT findings) fungal pneumonia with repetitively negative GM and G-glucan serum levels24 |
DM indicates diabetes mellitus; MI, myocardial infarction; GI, gastrointestinal; CXR, chest x-ray; GM, galactomannan; IPM, invasive pulmonary mucormycosis; and IPA, invasive pulmonary aspergillosis.
How do I establish the diagnosis of mucormycosis?
The clinical signs and symptoms of mucormycosis are nonspecific. Importantly, there are no biomarkers to indentify this disease. β-D-glucan test and Aspergillus galactomannan tests do not detect antigen components of the Mucorales cell wall.4 Therefore, a high level of suspicion in susceptible patient subgroups is of paramount importance (Table 1). Biopsy and culture from sterile sites are critical to distinguish mucormycosis from more common and more antifungal-sensitive molds, such as Aspergillus.1-4 Although thrombocytopenia and coagulopathy are common in hematologic malignancy patients,26 biopsy with platelet transfusion and fresh frozen plasma support should be considered, as the benefits of establishing a definitive diagnosis frequently outweigh the rather low bleeding risks in such patients.26,27 The yield of bronchoalveolar lavage is low for diagnosis of pathogens in hematology patients28 and early bronchoscopy (within 48 hours after the radiologic suspicion of disease) before extensive antifungal use might provide the best window of opportunity for a diagnosis.29 In a recent prospective study from a hematology unit in Austria with a high background rate of mucormycosis, an aggressive diagnostic approach that consisted of computed tomography (CT)–guided fine-needle aspiration and an array of polymerase chain reaction and antigen biomarkers improved the differentiation of mucormycosis from aspergillosis.30 Yet, 84% of leukemia and SCT patients were still receiving ineffective antifungal therapy for mucormycosis at the time of diagnosis.30
Although contamination of clinical specimens by the ubiquitous environmental Mucorales spores is always a possibility, the value of Mucorales-positive cultures (especially repetitive cultures) remains a critical indication of infection in immunocompromised patients.9 Unsurprisingly, the site of infection also has a major impact on the likelihood of histopathologic diagnosis.9 Sinuses are the major site of definitely identified infection because of their accessibility for biopsy.9 A variety of stains, including hematoxylin and eosin, Grocott-Gomori methenamine–silver nitrate, and periodic acid-Schiff, frequently will reveal characteristic hyphal elements in tissue.14 Histopathologic examination of infected tissue often shows characteristic broad (3-25 μm in diameter), ribbon-like, thin-walled, primarily aseptate hyphae that have irregular diameters; with nondichotomous irregular branching and accompanying tissue necrosis and fungal angioinvasion.1-4,14,31,32 Improved staining procedures with fluorescent stains, such as Calcofluour White/Blankofluor, may be more revealing with small numbers of hyphae or limited tissue samples.1-4,14 Paradoxically, even when fungal hyphae are seen in histopathologic analysis, fungal cultures are only positive in 50% of cases33 because of the friable nature of nonseptated hyphae, which are frequently damaged during tissue manipulation. Recovery of Mucorales from tissue can be improved by mincing (not homogenizing) tissue specimens1-4,14 and using culture techniques that simulate in vivo fungal growth, including incubation at 35°C to 37°C under relatively semianaerobic conditions.34
Communication with the clinical microbiology laboratory is essential in cases of suspected mucormycosis. Morphologic features of fungal identification, especially when assessed by persons with expertise in fungal identification, can provide a high level of accuracy comparable that of molecular methods.5,35 Although most Mucorales grow rapidly on standard media when incubated at 25°C to 30°C,1-4,14 they are sensitive to the protein inhibitor cycloheximide, which should be omitted from culture media to optimize recovery.
The importance of early differentiation of Mucorales from more common opportunistic molds, such as Aspergillus spp has generated considerable interest in development of culture- or histopathology-independent diagnostic tests, such as detection of specific antigens or nucleic acids using polymerase chain reaction or in situ hybridization techniques.36-38 Molecular techniques for detecting Mucorales are few, not widely available, and have not undergone rigorous clinical validation. Therefore, routine clinical use of polymerase chain reaction or other nonculture-based methods as a single approach for diagnosing mucormycosis cannot be recommended. Early and accurate diagnosis remains the most important barrier for improving survival in patients with mucormycosis.8 Unfortunately, a physician's early suspicion of Mucorales infection remains the most important element for early clinical diagnosis and effective preemptive treatment. Finally, it is important to remember that dual infections with other fungal pathogens may be encountered in high-risk hematologic malignancy patients (22% in a single institution observational study5 ), which can complicate the diagnosis and treatment of the infection.
Philosophy of care and management strategy
Mucorales have a strong tropism for invasion of blood vessels, resulting in tissue infarction and necrosis, the pathologic hallmark of mucormycosis (Figure 2).32 Early recognition and treatment of the infection are critical improving patient survival before angioinvasion, and necrosis become too extensive and the infection disseminates to other organs.4 Therefore, the management of mucormycosis in hematology patients is underscored by 3 key strategies: (1) rapid initiation of effective antifungal therapy and concomitant aggressive attempts for diagnosis, (2) extensive “early” surgical debridement of necrotic lesions, and (3) rapid control of underlying medical condition, when feasible.4 Small focal lesions should be surgically resected before they progress to involve critical structures or distal organs. Often the infection has an indolent clinical presentation until dissemination to other vital organs. Cooperation among the hematologists, infectious disease consultants, surgeons, pathologists, and microbiologists is essential in the early stages of infection to avoid unnecessary diagnostic and treatment delays that increase patient mortality.
Only 2 systemic antifungals are currently available with good Mucorales activity- amphotericin B (including the lipid formulations) and the triazole posaconazole.20 It is important to start one of these classes of antifungal agents as soon as possible, as treatment delays are associated with increased mortality.8 Echinocandins demonstrate modest activity against some Mucorales in vivo,39-41 which is enhanced when coadministered with lipid amphotericin B formulations.40,41 In a retrospective analysis of diabetic patients with rhino-orbital-cerebral mucormycosis, Reed et al noted that patients who received combination lipid amphotericin B-caspofungin therapy had significantly better treatment success and survival time compared with patients who received amphotericin B monotherapy.40
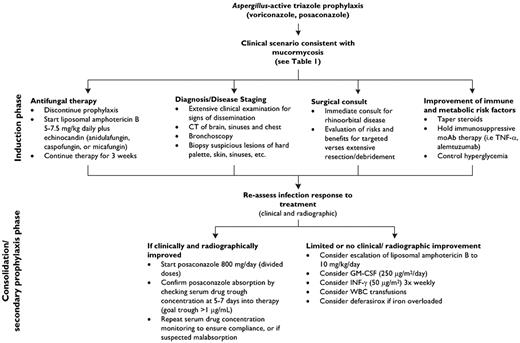
The choice of which antifungal to start should be made in parallel with attempts to establish a firm diagnosis (Figure 3). Because subclinical dissemination is common,9 the diagnostic strategy should include a thorough clinical examination and appropriate CT imaging of the brain, sinuses, and abdomen to “stage” the severity of infection and likelihood of dissemination. The latter is important for decisions of weighing the risk versus benefit of surgical debridement and/or debulking. In addition, some antifungals such as the echinocandins and posaconazole may have limit penetration into sanctuary sites of infections in patients with disseminated mucormycosis, including the central nervous system and eye.20 Initial decisions regarding the intensity of antifungal therapy often depend on the tempo of infection progression, the magnitude of clinical response in the first 7 to 10 days after initiation of appropriate therapy, and the underlying malignancy status of the patient, including plans for future chemotherapy or immunosuppression. In patients with adequate renal function, we recommend an “induction therapy” approach using intravenously administered antifungal agents with predictable pharmacokinetics (ie, lipid amphotericin B and echinocandins in combination) for the first 3 weeks, followed by a “de-escalation” strategy (Figure 3). The possible availability of an intravenous formulation in the coming years could make posaconazole a more appealing first-line option, as the drug's erratic bioavailability is the principle rate-limiting factor influencing attainment of therapeutic blood levels during the first week of therapy.42-46
A clinical approach to presumed and documented mucormycosis in the patient with hematologic disease.
A clinical approach to presumed and documented mucormycosis in the patient with hematologic disease.
The choice of which lipid amphotericin B formulation and the initial dose is an area of some confusion and debate. Most clinicians use a lipid formulation of amphotericin B with a usual starting dose of 5 mg/kg that is sometimes escalated to 7.5 to 10 mg/kg per day (liposomal amphotericin B) in an effort to control the infection. We consider amphotericin B lipid complex to be an effective alternative, although in our experience doses more than 5 mg/kg per day are less well tolerated than with the liposomal formulation of amphotericin B. High-dose liposomal amphotericin B carries a greater risk for nephrotoxicity and was not shown to be more effective than a 3-mg/kg daily dose in leukemic patients with invasive aspergillosis.47
Therefore, the decision to use higher doses of liposomal amphotericin B should be weighed against the patient's individualized risk to develop nephrotoxicity. Animal models of pulmonary mucormycosis have suggested that higher amphotericin B tissue concentrations may be required early during the infection for effective treatment of mucormycosis versus aspergillosis,48 which would be consistent with the relative tolerance in vitro of most Mucorales to the fungicidal effects of amphotericin B.14 Currently, a phase 2 clinical study of 10 mg/kg per day liposomal amphotericin B is underway for mucormycosis (www.clinicaltrials.gov/ct2/show/NCT00467883). We do not use amphotericin B deoxycholate because of its frequent and rapid onset of renal toxicity in hematology patients with opportunistic mycoses.41
Step down therapy (consolidation therapy, secondary prophylaxis)
Eventually, patients who respond to a parenteral lipid amphotericin B-based treatment, given for at least 3 weeks, are transitioned to oral posaconazole as maintenance/secondary prophylaxis with serial monitoring of posaconazole serum drug concentrations to ensure compliance and absorption of the antifungal. Serum blood levels of posaconazole are often quite variable in hematologic malignancy patients42-46 because of erratic absorption of the drug, which is especially problematic in patients with mucositis, graft-versus-host disease of the gut, poor appetite and/or nausea, diarrhea, or in patients receiving potent acid suppression therapy.45 It is common for hematologic malignancy patients to have multiple risk factors for malabsorption, frequently leading to undetectable serum concentrations and increased risk of breakthrough infection.42,44,49 Indeed, one-third of patients at the M. D. Anderson Cancer Center who do not have mucositis or gut graft-versus-host disease still have suboptimal plasma concentrations of posaconazole with maximal doses, emphasizing that suboptimal pharmacokinetics cannot be predicted just on the basis of clinical patient characteristics alone (D.P.K., unpublished data, 2010). Unfortunately, an intravenous formulation of posaconazole is not currently available, and absorption of the drug is limited at 800 mg/d when administered with food in divided doses (2-4 times daily). Therefore, current strategies to improve posaconazole serum concentrations are focused primarily on improving drug dissolution and absorption (ie, administer with high-fat food, acidic beverage), discontinuing acid suppression therapy (especially proton pump inhibitors), and serum drug level monitoring. Patients prescribed posaconazole should be provided education and counseling by the physician, nurse, or pharmacist and be seen by the nutritionist to develop a personalized nutritional plan that will facilitate compliance and drug absorption.50
We monitor serum posaconazole trough drug concentrations after 7 days to therapy42 to ensure that drug levels are at least 1 μg/mL (ideally > 2 μg/mL as minimal inhibitory concentration (MIC) 90 for Mucorales is > 1 μg/mL51 ) and periodically thereafter with random serum levels. Low or undetectable serum concentrations are addressed by trying to improve the absorption of the drug rather than escalating posaconazole daily dosages > 800 mg/d, which paradoxically often results in diminished blood levels.46 Drug interactions can be problematic with posaconazole, even though the drug is not a major substrate of the cytochrome P450 system. Concomitant administration of posaconazole with drugs that accelerate CYP P450 and phase 2 (uridine diphosphate glucoronidation) metabolism, such as rifampin and rifamycins, reduces posaconazole exposures by nearly 50%.44 Posaconazole is also a potent inhibitor of CYP enzymes, including CYP 3A4, which results in numerous and sometimes unpredictable drug interactions with most immunosuppressive agents (eg, cyclosporine, tacrolimus, sirolimus) and chemotherapy (eg, cyclophosphamide, busulfan, Vinca alkaloids) used in transplantation.44 These drug interactions can be especially problematic in patients who require repeated exposures to drug of concern (ie, many acute lymphocytic leukemia and lymphoma chemotherapy regimens) as the long half-life of posaconazole necessitates “washout periods” of 3 to 4 days, which could delay chemotherapy. Acid suppression therapy, particularly cimetidine and proton pump inhibitors (eg, omeprazole esomeprazole), reduce the posaconazole maximum concentration and area under the curve by 30% to 40%, which may jeopardize therapy in patients with concomitant underlying risk factors for drug malabsorption.42-46 Patients with persistently low or undetectable serum drug levels, poor oral intake, or gastrointestinal dysfunction (severe nausea, diarrhea) often must be transitioned back to intravenous liposomal amphotericin B, even in the absence of obvious disease if they are receiving additional immunosuppressive therapy.
Because of their ability to produce prolonged tissue concentrations, we occasionally use intermittent doses of lipid amphotericin B (ie, 5 mg/kg 3 times per week or possibly even 2 times per week) after at least 3 to 4 weeks of initial therapy as an alternative to posaconazole therapy. These intermittent dosing strategies also provide a more facile transition to outpatient, posaconazole-based oral therapy. We frequently overlap reduced dosages of lipid amphotericin B with posaconazole for 10 to 14 days before the transition to posaconazole monotherapy so posaconazole absorption can be documented before lipid amphotericin B is discontinued. If posaconazole concentrations are low (< 500 ng/mL), then we consider continuing lipid amphotericin B dosing to at least 1 to 2 times weekly in patients who are clinically stable until posaconazole absorption can be improved. If a patient has severe diarrhea, ongoing mucositis, or compliance issues, we do not attempt to transition to posaconazole. Typically, posaconazole can be continued safely for months to years depending on the immune status of the patient. However, we have observed occasional patients reactivating with mucormycosis during subsequent chemotherapy cycles after more than 1 year of posaconazole secondary prophylaxis with “adequate” serum concentrations (D.P.K., unpublished data, 2010).
Antifungal MICs and synergy?
Data on the antifungal susceptibility of Mucorales spp are limited, and MIC testing remains investigational.4 The MIC endpoints for these rapidly growing fungi are inconsistent, not standardized, and at times, difficult to interpret. Because interpretive MIC break points for Mucorales have yet to be defined, the correlation between clinical responses and MIC values is uncertain. Mucorales are resistant in vitro to many antifungals,51 including flucytosine, ketoconazole, fluconazole, voriconazole, and the echinocandins. In addition, they have variable susceptibility to itraconazole.51 Amphotericin B and posaconazole are the most active agents in vitro,20,51 although their activity varies among different Mucorales families.51 Animal models39,41,52 and limited clinical experience8,40,41 suggest that combinations of lipid amphotericin B and echinocandins are more effective than lipid amphotericin B alone; however, this concept has not been formally tested in a randomized controlled trial. Currently, there are no in vivo data to suggest that combinations of posaconazole with echinocandins or liposomal amphotericin B are antagonistic against Mucorales.
Salvage therapy
Small uncontrolled salvage studies have suggested a response rate of approximately 40% for both posaconazole53,54 and with amphotericin B lipid complex.55 However, multiple selection biases associated with the survivors of opportunistic mycoses in salvage studies precludes confident conclusions on the merit of these agents. Furthermore, assessing failure of antifungal therapy requires careful judgment.56 For example, it is common for patients with invasive mucormycosis to have concomitant bacterial and viral infections, often with drug-resistant organisms (eg, Pseudomonas aeruginosa). In addition, noninfectious pulmonary processes, such as bronchiolitis obliterans, diffuse alveolar hemorrhage, drug toxicity, heart failure, and even leukemic infiltration, in the lungs could coexist and create difficulties in assessing therapy response and failure. Therefore, worsening of respiratory status may represent emergence of a bacterial superinfection or viral pneumonitis or worsening of a preexisting noninfectious pulmonary condition, rather than progression of the underlying fungal infection. Second, initial “paradoxical” worsening pulmonary lesions could occur in patients with opportunistic mycoses, especially in the setting of neutrophil recovery and may be mistaken for failure of antifungal therapy.57 Increasing the dose of the liposomal amphotericin B (7.5-10 mg/kg per day), adding an echinocandin or posaconazole, and switching to posaconazole are reasonable strategies for patients with progressive infection on lipid amphotericin B therapy alone. For patients who develop renal toxicity to lipid amphotericin B, changing lipid amphotericin B to every 48 to 72 hours, adding posaconazole, or switching to posaconazole altogether therapeutic drug monitoring are approaches used in individual situations.
Surgery
Surgical debridement of isolated cutaneous or sinus lesions is crucial and must be done without delay because of the aggressive nature of the infection.4 Repeated removal of necrotic tissue or aggressive surgical measures, such as enucleation of the eye may be required to prevent dissemination.1-4 Decisions regarding the iming and extent of debridement are often made at bedside. A CT or MRI scan before surgery and intraoperative frozen section analysis help determine the extent of tissues and tissue margin involvement. Surgery in conjunction with systemic antifungal therapy has been shown to significantly improve survival rates in pulmonary mucormycosis,58 realizing that the reported outcomes may reflect a selection bias of patients with better underlying performance status and leukemia prognosis of the underlying disease.58 Frequently, extensive disfiguring debridement of the sinuses and orbit requires subsequent plastic surgery to cosmetically address anatomic defects.59
In less-severely ill patients with unifocal pulmonary mucormycosis, early surgical removal of infected or devitalized tissue when the infection is localized is reasonable and should provide the greatest benefit.60 Lobectomy is often adequate in most patients, but pneumonectomy may be necessary for proximal or extensive involvement.58 The benefit of pulmonary resection is unknown in patients with multifocal or disseminated mucormycosis.
Management of comorbidities
Beyond tapering immunosuppressive therapy, many comorbidities present in extensively treated hematologic malignancy patients and are known to enhance the susceptibility to mucormycosis. Hyperglycemia, in particular, and ketoacidosis are known to impair neutrophil function and killing activity against Mucorales hyphae.1-4 Diabetic ketoacidosis increases the availability of free iron available to Mucorales and is the most frequent underlying risk factor for mucormycosis in the developing world.1-4,14 Hyperglycemia associated with long-term high-dose corticosteroid therapy is a key late risk factor for breakthrough mucormycosis,4 although it is unknown whether tighter glycemic control reduces this risk. There is no validated approach on the best way to taper immunosuppression. This reflects the complex and pleiotropic effects (concomitant or sequential) of the various immunosuppressive agents to the immune system. Specifically, the rapidity of corticosteroid tapering as it relates to meaningful survival benefits is unknown61 but should be attempted nonetheless in patients with active infection.
Iron overload
Rhizopus oryzae, the most common Mucorales species isolated from cancer patients with pulmonary mucormycosis,1,62-64 uses free iron as growth factor. Older iron chelators, such as deferoxamine, paradoxically increased the susceptibility of patients to mucormycosis because of the ability of the fungus to use this chelator as a xenosiderophore to acquire free iron in vivo. Reassuringly, newer iron chelators (eg, deferasirox) are not associated with increased risk of mucormycosis.65 Indeed, deferasirox exhibits direct fungicidal effects against Mucorales in vitro and in animal models via iron starvation. Interestingly, preclinical data support addition of deferasirox to initial lipid polyene therapy, particularly for central nervous system infection. Deferasirox has been administered as open-label therapy to patients with mucormycosis, either in the setting of initial or salvage combination therapy, with generally favorable results. However, aside from these small numbers of published cases,41 virtually all of the clinical data available for deferasirox is in the long-term treatment of chronically iron-overloaded patients. Specifically, the safety of adjunctive deferasirox has not been established in acutely ill patients with mucormycosis. A clearer understanding of the feasibility and safety profile of initial lipid polyene-deferasirox therapy will be offered by the ongoing phase 2 clinical (DEFEAT MUCOR trial; NCT00419770). Until the results of that study are available, echinocandins may be preferred combination agents for patients on lipid amphotericin B products because of the enhanced potential for nephrotoxicity with deferasirox. The potential for triple therapy with lipid polyenes, echinocandins, and deferasirox has also demonstrated some promise in animal infection models.41
Adjunct treatments
Hyperbaric oxygen therapy is a beneficial adjunct therapy for mucormycosis, particularly diabetic patients with rhinocerebral or extensive cutaneous disease.66 Specifically, the increased partial pressure of oxygen achieved with hyperbaric therapy seems to improve neutrophil activity and oxidative killing of amphotericin B. In addition, high concentrations of oxygen can inhibit the growth of Mucorales in vitro and improve the rate of wound healing by increasing the release of tissue growth factors.67 However, this treatment has not been studied vigorously to determine efficacy and cannot be routinely recommended. Immune augmentation strategies are also considered in patients with refractory mycormycosis, including administration of cytokines (eg, granulocyte-macrophage colony-stimulating factor, interferon-γ).68-71 In select neutropenic patients, granulocyte transfusion may be a useful bridge until neutrophil recovery, although the clinical benefit remains unproven; and serious adverse effects, including pulmonary toxicity and accelerated cavitation/bleeding, have been reported in patients with opportunistic lung mycoses. Therefore, there are limited data to support the routine use of these adjunct strategies at this time.
How long of treatment is long enough?
In general, antifungal therapy of mucormycosis should be highly individualized and continued until there is clinical resolution of signs and symptoms of infection. At a minimum, patients should have resolution of radiographic signs of active disease on serial imaging, with the possible exception of radiographic findings, which are thought to be the result of postinflammatory or postoperative scar formation. Positron emission tomography CT may be useful in making this distinction in selected patients.72 Patients should also have negative follow-up cultures and reversal of underlying immunosuppression. Because we have observed late recurrences of mucormycosis in patients who were previously successfully treated but required subsequent chemotherapy, our threshold for discontinuing antifungals is relatively high.
How to monitor for relapse of mucormycosis
There are no validated approaches or biomarker to monitor for clinical relapse. Intensification of immunosuppression, metabolic derangement (eg, uncontrolled hyperglycemia), leukemia relapse, cytomegalovirus reactivation, drug-related issues (eg, noncompliance, drug interactions), and the presence of anatomic sequestra are factors that increase the risk of recurrent infection. As mentioned before, attributing worsening of pulmonary lesions to known preexisting pulmonary mycormycosis is sometimes difficult. For example, in patients with chronic pulmonary cavitary mucormycosis, intercurrent superinfection may develop in the lung cavities. Appropriate cultures and biopsies should be pursued, especially if the scenario is atypical for recrudescence of mucormycosis. In small series, white blood cell transfusion as secondary prophylaxis has been tried in selected high-risk patients with severe mold infections who were at risk of relapse of their mycoses.73
Prognosis
As in all opportunistic mycoses, the key factors determining the prognosis of invasive mucormycosis are the site of infection and underlying host factors, particularly the underlying status of hematologic disease and immunosuppression.8,74 In a series of 391 patients with hematologic malignancies with invasive fungal infections, Pagano et al reported that most patients who developed mucormycosis died within 12 weeks of diagnosis in contrast to patients with aspergillosis who had a significantly better prognosis.75 In addition, some unusual Mucorales are associated with poor overall prognosis.76 In the large series by Roden et al,2 infection with Cunninghamella spp was associated with a 2.78 fold-increase in the risk of death (95% CI, 1.11-6.96; P = .029) compared with more common Rhizopus species.2 This poorer outcome may be in part the result of the inherently greater resistance of Cunninghamella species to antifungal agents and polymorphonuclear leukocyte-mediated hyphal damage.76
Prevention, prophylaxis, and infection control
The spectrum of posaconazole includes covers both Aspergillus and Mucorales, which should theoretically reduce the risk of mucormycosis cases in high-risk patients when administered as prophylaxis. However, posaconazole is not a panacea.77 Compliance issues, suboptimal absorption, and drug-drug interactions frequently result in low posaconazole serum levels and occasional breakthrough mucormycosis infections.49 It is also unclear whether posaconazole use and suboptimal pharmacokinetics are driving forces behind culture-negative breakthrough mucormycosis. In view of the ever low autopsy rates,13 the lack of culture-independent serum-based surrogate diagnostic markers and prospective surveillance of trends in epidemiology of various opportunistic fungal infections, the answer may never be known. Given the increasingly ambulatory nature of patients at risk for mucormycosis, patients should be repeatedly reminded of healthy living practices after SCT or leukemia therapy,78 especially with respect to avoiding activities associated with exposures to high aerosolized inoculum of mold spores and trauma. Finally, meticulous attention to infection control practices, including policies during construction and renovation of hospital facilities, and early recognition of nosocomial outbreaks and pseudo-outbreaks is critical.8
Future directions
Many challenges must be overcome to improve overall outcomes associated with invasive mucormycosis. The immunopathogenesis of mucormycosis is poorly understood, and the traditional animal models are imperfect and logistically difficult. No immunogenetic risk factors for mycormycosis have been described in humans, and this is a fertile area for further investigation. As traditional79 as well as alternative experimental models,80 as well as genome sequencing81 and molecular tools for studying the Rhizopus biology82 are rapidly expanding, it is anticipated that there will be an acceleration of discovery of new, traditional or even immunopharmacologic83 targets or possibly even vaccine development. Second, the advancement of fungal diagnostics with the implementation of novel fungal biomarkers is a formidable frontier in mucormycosis. This is an area full of difficulties and promise for innovative approaches. Finally, improved risk stratification of patients susceptible to mucormycosis based on time-honored host characteristics (eg, severity and persistence of underlying immunosuppressing condition, prior antifungal selection pressure, metabolic impairment, quantitative immune defects, extremes of age, poor performance status) should lead to individualized approach to prophylaxis, early diagnostic platforms and treatment of this uncommon, yet emerging and frequently devastating opportunistic mycosis.
Acknowledgments
The authors thank Cheryl Perego for the institution infection surveillance data.
D.P.K. was supported by the M. D. Anderson Cancer Center Faculty E. N. Cobb Scholar Award Research Endowment and the National Institutes of Health (M. D. Anderson Cancer Center support grant CA016672).
National Institutes of Health
Authorship
Contribution: D.P.K. wrote the manuscript; and R.E.L. revised the manuscript and created the figures.
Conflict-of-interest disclosure: D.P.K. has received research support and honoraria from Merck & Co Inc, Fujisawa, Enzon, Pfizer, and Schering-Plough. R.E.L. has received research support from Merck & Co Inc.
Correspondence: Dimitrios P. Kontoyiannis, Department of Infectious Diseases, Infection Control and Employee Health, Unit 402, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: dkontoyi@mdanderson.org.