Leukemia occurs as the result of leukemia-promoting factors and the ability/inability of the immune system to attack and eliminate upcoming malignant cells. In this issue of Blood, Almalte et al describe in a case-control study how the presence of activating killer-cell immunoglobulin-like receptors (KIRs) reduces the risk of childhood leukemia.1
“Why me?” is a common question of families with children diagnosed with leukemia and we usually have no satisfying answer. Childhood leukemia is thought to be associated with genetic factors, and investigators have focused on leukemia-promoting factors in the past. However, the development of leukemia could not only be a result of malignant cell growth but also depend on the ability/inability of the immune system to attack and eliminate the initial malignant cells. In a candidate gene approach a novel association between activating KIR genes and childhood leukemia provides insights concerning pathogenesis of childhood leukemia and has implications for developing new immunotherapies for this cancer.1
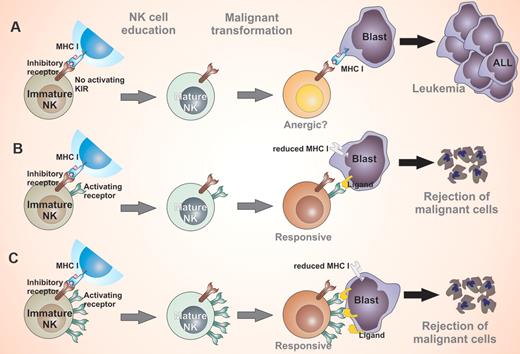
Childhood leukemia is thought to be associated with genetic factors, and investigators have focused on leukemia-promoting factors in the past. However, the development of leukemia could be a result of the inability of the immune system to attack and eliminate upcoming malignant cells (A). In the presence of KIR genes, the risk of childhood leukemia is reduced because of a possible attack of leukemia cells by NK cells or T cells (B). Inheriting a higher number of activating KIR genes (≥ 4) is associated with significant reductions in risk for ALL in children (C).
Childhood leukemia is thought to be associated with genetic factors, and investigators have focused on leukemia-promoting factors in the past. However, the development of leukemia could be a result of the inability of the immune system to attack and eliminate upcoming malignant cells (A). In the presence of KIR genes, the risk of childhood leukemia is reduced because of a possible attack of leukemia cells by NK cells or T cells (B). Inheriting a higher number of activating KIR genes (≥ 4) is associated with significant reductions in risk for ALL in children (C).
KIRs are a group of receptors expressed on natural killer (NK) cells and T-cell populations. KIRs belong to the Ig-superfamily and consist of type 1 transmembrane glycoproteins with 2 or 3 Ig-like domains and possess either a short (activatory KIR) or long (inhibitory KIR) cytoplasmic tail. The overall KIR repertoire is determined by the KIR genotype and the decision as to which KIRs are expressed on each NK cell is regulated by the methylation of KIR gene loci. KIR receptors specifically bind HLA class I molecules (HLA-A, -B, and -C) and structurally it has been shown that KIRs bind the peptide-binding region of HLA. The ligands of activatory KIRs are believed to belong to stress-induced, viral, or tumor proteins. Unlike T cells, which respond to foreign peptides on HLA molecules, NK cells attack cells that are “missing self,” that is, lacking HLA molecules through loss of inhibition.2 In addition to sensing a loss of HLA class I in target cells, full effector function of NK cells requires triggering of their activating receptors on virus-infected cells or malignant cells. Although ligation of inhibitory receptors during NK-cell maturation has been well described as license for functional maturation called NK-cell education,3 the role of activatory receptors remains elusive.
Of the 6 distinct activating KIR genes, humans may inherit different numbers of these genes. Little is known about the impact of this genetic variation on innate susceptibility/resistance of humans to developing acute lymphoblastic leukemia (ALL). Almalte et al address this issue by performing a case control study in Canadian children of white origin.1 Their results show that the presence of activating KIRs reduces the risk of childhood leukemia. Of the 6 activating KIR genes, KIR2DS2 is maximally associated with decreased risk for the disease. Harboring activating KIR genes is associated with reduced risk for developing pre-B ALL in these children. Furthermore, this study shows that inheriting a higher number of activating KIR genes is associated with significant reductions in risk for ALL in children, including different phenotypes of B-cell and T-cell ALL. This study provides evidence of how the immune system may attack upcoming malignant cells and thereby prevent the onset of childhood leukemia. This concept is supported by the fact that genes encoding KIRs and their HLA class I ligands segregate independently; thus, some individuals may express a KIR gene but not its cognate ligand.
In a prospective study among patients with lymphoma or solid tumor, patients with KIR-HLA receptor ligand mismatch had a low risk of relapse after an autologous hematopoietic stem cell transplantation.4 In the past decades allogeneic stem cell transplantation has become a standard treatment for high-risk childhood leukemia, and graft-versus-leukemia effects have been part of this treatment approach. However, relapse remains the major problem after stem cell transplantation. Current concepts of NK-cell education include the plasticity for developmental reprogramming.5 This means that NK cells could be tuned to graft-versus-malignancy effects by change of environment. In addition, selection of haploidentical stem cell donors according to KIR alloreactivity using KIR ligands has shown improved survival of adult stem cell recipients with acute myeloid leukemia6 but not adult ALL. This concept has been further developed using a receptor ligand model adapted to childhood ALL.7 Childhood ALL may differ from adult ALL in that HLA I expression is reduced in childhood ALL,8 supporting NK-cell alloreactivity.9 Recently, beneficial outcome of HLA-mismatched haploidentical stem cell transplantation in childhood leukemia has been attributed to graft-versus-leukemia effects.10
This study by Almalte et al suggests that activating KIRs are an important factor for the immunologic attack against leukemia cells and may be applicable to immunotherapies after stem cell transplantation.1 However, the mechanisms of activating KIRs in the immune response and graft-versus-leukemia reactions still remain to be investigated. So our answer to the parents with childhood leukemia is still not satisfying, but we might be able in the future to prevent relapse by improving the immune response against malignant disease.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal