Abstract
In the STOP II trial, discontinuation of prophylactic transfusions in high risk children with sickle cell disease (SCD) resulted in a high rate of reversion to abnormal blood-flow velocities on transcranial Doppler (TCD) ultrasonography and strokes. We analyzed data from STOP II to determine the effect of discontinuing transfusions on the development or progression of silent brain infarcts on magnetic resonance imaging (MRI). At study entry, 21 of 79 (27%) patients had evidence of silent infarcts. There were no statistically significant differences in baseline characteristics between patients with normal brain MRI or silent infarcts at study entry. At study end, 3 of 37 (8.1%) patients in the continued-transfusion group developed new brain MRI lesions compared with 11 of 40 (27.5%) in the transfusion-halted group (P = .03). The total number of lesions remained essentially unchanged decreasing from 25 to 24 in the continued-transfusion group while increasing from 27 to 45 in transfusion-halted patients. Thus, discontinuation of transfusions in children with SCD and abnormal TCD who revert to low-risk increases the risk of silent brain infarction. Together with data from STOP, these findings demonstrate that transfusions prevent the development of silent infarcts in patients with SCD and abnormal TCD but normal MRA.
Introduction
Estimates of the prevalence of silent brain infarcts, in children with sickle cell disease (SCD) range from 17% to 35%.1-4 Despite the terminology, ‘silent infarcts’ are clinically significant given their association with subsequent overt stroke and neurocognitive deficits in school-aged children.2,5-9 Better understanding of the associated risk factors and efforts to optimize prevention remain essential. Reported risk factors for silent infarction are raised white blood-cell count, anemia, severe vasculopathy, and the SEN β-globin haplotype.4,10,11
Transfusions have been successfully used to prevent initial or recurrent strokes in SCD.12-16 To prevent first strokes, the Stroke Prevention Trial in Sickle Cell Anemia (STOP) used prophylactic red-cell transfusions in children who were identified by transcranial Doppler (TCD) ultrasonography as being at high risk for stroke. This strategy reduced the incidence of stroke among such children from 10% per year to < 1% per year.15 The STOP study led to recommendations for TCD screening and prophylactic transfusion for children with abnormal velocities on ultrasonography.17 Among patients with MRI evidence of silent infarction and raised velocities by TCD enrolled in STOP, transfusion also diminished the risk of new silent infarcts.18 Despite the reduced risk of stroke, the potentially indefinite duration of transfusion aroused concern about adverse effects, especially iron overload. In the Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP II) Trial, discontinuation of transfusions in patients whose TCD velocities had normalized (normal TCD) resulted in a high rate of reversion to abnormal blood-flow velocities (abnormal TCD) or stroke.19
In this study, we analyzed STOP II data to determine the effect of discontinuing transfusions on the development or progression of silent brain infarcts on magnetic resonance imaging. The STOP II trial excluded patients with severe artery stenosis detected on MRA thus eliminating a known risk factor for silent infarcts4 and this allowed us to evaluate whether the occurrence of these infarcts is associated with other factors such as higher rates of hemolysis.
Methods
Subjects
The STOP II trial has been described in detail.19 Briefly, children with SCD who had a high risk of stroke on the basis of a TCD screening examination and who had received transfusions for 30 months or longer, during which time the TCD readings became normal, were evaluated. Children with severe stenotic lesions on cerebral magnetic resonance angiography (MRA) were excluded. The children were randomly assigned to continued transfusion or cessation of transfusion. The composite primary end point of the STOP II trial was stroke or reversion to a result on TCD examination indicative of a high-risk of stroke. The protocol was approved by the institutional review boards at the participating institutions. Written informed consent was obtained from a parent or guardian of the child in all instances, and the children's assent was obtained, when appropriate, in accordance with the Declaration of Helsinki. The study was stopped after 79 children of a planned enrollment of 100 underwent randomization.
MRI studies of the brain
MRI of the brain was required before patients underwent randomization (for patients with multiple MRIs the closest to study entry was evaluated), annually, at the time of suspected neurologic events, and at closure of the study (exit MRI). If a patient had an annual or an event MRI within 4 months of study closure, this examination was counted as an exit MRI and a new exit MRI was not required. MRIs referred to as “study end MRI” in this report refer to exit MRIs. The study protocol included axial T1-weighted spin–echo images (repetition time, 400-800 milliseconds, and echo time, 10-30 milliseconds). Axial spin–echo and fluid-attenuated inversion recovery (FLAIR) T2-weighted images, spin–echo or fast (turbo) spin–echo images with slices at a thickness of 5 mm, coronal spin–echo and FLAIR images (with the use of the same protocol as axial), and diffusion-weighted imaging (gradient strength, B 1000), with axial images in the x, y, and z planes, were performed. All images were reviewed for the presence, size, and location of ischemic lesions by 2 experts who were unaware of the treatment assignment. Review was done independently, and in case of disagreement, the 2 experts reviewed the images jointly until a consensus was reached. When annual studies or those obtained at the time of a clinical event were read, they were compared with previously obtained images. Stroke was defined as persistent abnormalities or transient neurologic symptoms accompanied by a new cerebral lesion appropriate to the patient's clinical presentation. Silent infarcts were defined by evidence of cerebral infarction on MRI in patients without a compatible history of a cerebrovascular event. In this study, patients were stratified at randomization according to presence or absence of silent infarcts on MRI.
Laboratory studies
Complete blood count, reticulocyte count, quantitative hemoglobin electrophoresis, and alloantibody screening were performed before each transfusion and quarterly. Levels of serum ferritin as well as alanine aminotransferase, γ-glutamyltransferase, lactate dehydrogenase (LDH), and bilirubin levels were measured at the core laboratory (at the Medical College of Georgia in Augusta). Screening for hepatitis B and C viruses were performed annually.
Statistical analysis
Baseline characteristics including demographics and clinical laboratory values for patients with and without silent infarcts at study entry were compared using the Student t test for continuous variables and the Fisher exact test for categorical variables. The number of lesions was compared at study entry and at study end for each patient to determine newly developed lesions during the study by treatment group. To compare the “no-change-lesion” group to the “newly-developed-lesion” group with respect to laboratory variables, as well as STOP II primary end points (development of stroke or abnormal TCD) the Student t test was used for continuous variables and the Fisher exact test for categorical variables. All reported P values are 2-sided and were not adjusted for multiple testing. The level of significance was set at < .05. The analysis was performed based on intent-to-treat in the different arms.
Results
Of the 79 children randomized, 38 (48.1%) were assigned to the continued-transfusion and 41 (51.9%) to the transfusion-halted groups. At study entry, 21 (27%) patients had evidence of silent infarcts on brain MRI. A total of 52 lesions were identified in the 21 patients (25 in the continued-transfusion and 27 in the transfusion-halted groups). Nine subjects had 1 lesion, 3 had 2, and 9 (42.9%) had 3 or more lesions at study entry. Lesions were most common in the deep white matter or the periventricular area in the frontal and/or parietal lobes. No lesions were seen in the internal capsule, brainstem, or cerebellum, but 7 occurred in the basal ganglia or thalamus. Lesion size varied with 30 characterized as “small punctate” areas a few millimeters in size, 18 “medium ovoid” (0.5-1.5 cm in largest diameter), and 4 “large” (> 1.5 cm).
Patients with normal brain MRI included 28 (73.7%) and 30 (73.2%) patients from the continued-transfusion and transfusion-halted groups, respectively; reflecting planned stratification. There were no statistically significant differences in baseline characteristics between patients who had normal brain MRI or silent brain infarcts at study entry (Table 1).
Characteristics of patients at study entry
| Characteristic . | Normal MRI . | Silent infarct . | P . |
|---|---|---|---|
| n (%) | 58 (73.4) | 21 (26.6) | |
| Age, y | 12.1 ± 0.43 | 12.6 ± 0.67 | NS |
| Male, n (%) | 25 (43.1%) | 8 (38.1%) | NS* |
| Hemoglobin, g/dL | 9.2 ± 0.35 | 9.7 ± 0.29 | NS |
| White cell count, ×10−3/mm3† | 11.4 ± 0.62 | 11.1 ± 0.81 | NS |
| Reticulocyte count, %† | 7.3 ± 0.59 | 8.2 ± 1.29 | NS |
| Platelet count, ×10−3/mm3‡ | 357 ± 18.0 | 387 ± 23.3 | NS |
| Hemoglobin S, %§ | 19.9 ± 1.3 | 20.2 ± 2.1 | NS |
| Indirect bilirubin, mg/dL‖ | 2.58 ± 0.24 | 2.55 ± 0.25 | NS |
| LDH, IU/l§ | 477 ± 34.8 | 419 ± 34.0 | NS |
| Four α-globin genes | 41 (70.7%) | 19 (90.5%) | NS* |
| TCD, cm/sec¶ | 212 ± 1.3 | 220 ± 4.4 | NS |
| Characteristic . | Normal MRI . | Silent infarct . | P . |
|---|---|---|---|
| n (%) | 58 (73.4) | 21 (26.6) | |
| Age, y | 12.1 ± 0.43 | 12.6 ± 0.67 | NS |
| Male, n (%) | 25 (43.1%) | 8 (38.1%) | NS* |
| Hemoglobin, g/dL | 9.2 ± 0.35 | 9.7 ± 0.29 | NS |
| White cell count, ×10−3/mm3† | 11.4 ± 0.62 | 11.1 ± 0.81 | NS |
| Reticulocyte count, %† | 7.3 ± 0.59 | 8.2 ± 1.29 | NS |
| Platelet count, ×10−3/mm3‡ | 357 ± 18.0 | 387 ± 23.3 | NS |
| Hemoglobin S, %§ | 19.9 ± 1.3 | 20.2 ± 2.1 | NS |
| Indirect bilirubin, mg/dL‖ | 2.58 ± 0.24 | 2.55 ± 0.25 | NS |
| LDH, IU/l§ | 477 ± 34.8 | 419 ± 34.0 | NS |
| Four α-globin genes | 41 (70.7%) | 19 (90.5%) | NS* |
| TCD, cm/sec¶ | 212 ± 1.3 | 220 ± 4.4 | NS |
Plus-minus values are means ± SE. P-values were calculated by Student t-test, except where indicated otherwise.
MRI indicates magnetic resonance imaging; LDH, lactate dehydrogenase; TCD, transcranial Doppler ultrasonography; and NS, not significant (P ≥ .05).
The value was calculated by Fisher exact test.
Two patients in the transfusion-halted group were excluded, one because no laboratory values were available at study entry and the other because the blood sample was too old to be processed.
Four patients were excluded, 1 from the continued-transfusion group because the blood sample was clotted and could not be processed, and 3 from the transfusion-halted group (1 because no blood sample was available at study entry, 1 because the blood sample was too old to be processed, and 1 because the blood sample was clotted).
Laboratory values at study entry were not available for 1 patient in the transfusion-halted group. LDH normal reference range is 120-246 IU/l.
Two patients were excluded, one in the transfusion-halted group because laboratory values at study entry were not available and the other in the continued-transfusion group because of missing data. To convert values for bilirubin to micromoles per liter, multiply by 17.1.
The value is the average of 2 qualifying TCD examinations performed before transfusion that showed abnormal velocities or 1 TCD examination if the velocity was < 220 cm/second (for new patients entering the STOP II trial).
New brain MRI lesions
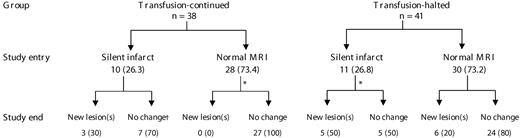
There was no statistically significant difference in the mean follow-up time of patients in the continued-transfusion (464 days) and transfusion-halted (523 days) groups. At study end, 3 of 37 (8.1%) patients in the continued-transfusion group developed new brain MRI lesions compared with 11 of 40 (27.5%) in the transfusion-halted group (P = .03; 1 patient in each group had no follow-up brain MRI; Figure 1). Of the 14 patients who developed new brain MRI lesions, 5 (35.7%) patients developed multiple new lesions (all in the transfusion-halted group) while 9 (64.3%) developed single new lesions. The total number of lesions at study end was 69. In the continued-transfusion group the total number of lesions remained stable (decreased from 25 to 24, 1 patient actually reverted to a normal scan). In the transfusion-halted group, the total number of lesions increased from 27 to 45. Among patients with normal brain MRI at study entry, 27 of 28 patients in the continued-transfusion group remained lesion-free (1 did not have follow-up brain MRI) compared with 24 of 30 patients in the transfusion-halted group (P = .024).
Brain MRI findings in the study population at study entry and study end. Data presented as n (%). *One patient had no follow-up MRI. †Three patients actually had a decrease in the number of lesions, 1 reverting back to a normal scan.
Brain MRI findings in the study population at study entry and study end. Data presented as n (%). *One patient had no follow-up MRI. †Three patients actually had a decrease in the number of lesions, 1 reverting back to a normal scan.
Predictors of new brain MRI lesion formation in transfusion-halted patients
There were no statistically significant differences in white cell counts, platelet counts, fetal hemoglobin concentrations, and serum ferritin levels for patients who developed new brain MRI lesions compared with patients with no change; in both subgroups of patients with normal and abnormal brain MRI at study entry. In the subgroup with normal brain MRI at study entry, all patients who developed new brain MRI lesions had 4 α-globin genes (6 of 6) compared with 74% (17 of 23) in patients who remained lesion-free (P = .160). Data regarding markers of hemolysis were difficult to interpret.
Correlations with stroke, abnormal TCD, and stenosis on MRA
Among the 41 children in the transfusion-halted group, high-risk TCD results developed in 14 and stroke in 2 others. Neither of these events of the composite end point occurred in the 38 children who continued to receive transfusions.19 In transfusion-halted patients, there was no statistically significant correlation between the development of new silent infarcts and conversion to high-risk TCD or stroke. Four of the 11 patients (36.4%) who had new lesions in the transfusion-halted group had a positive composite end point (high-risk TCD or stroke), compared with 12 of 29 patients (41.4%) who had no change on study end MRI (P = .772).
In STOP II, MRA scans at study end were available for 78 of 79 patients. Only 2 of 78 patients, both in the transfusion-halted arm, had evidence of stenosis on MRA at study end. The first patient had 2 MRI lesions at study entry and 5 new lesions at study end. He also developed a stroke. The second patient had 1 MRI lesion at study entry and developed 2 new lesions at study end. The remaining 76 patients (97%) had essentially unchanged MRA findings (no stenosis).
Discussion
Our study demonstrates that discontinuing transfusion in children with SCD, who revert to low-risk on TCD after long term transfusion, is not only associated with a higher occurrence of overt stroke and transformation to high-risk TCD, but also more frequent development of silent brain infarction. Transfusions were protective in both patients with normal and abnormal brain MRI at inclusion. In some patients, continuing transfusion was also associated with a decrease in the number of brain lesions; this may probably represent chance variations in partial volume effect affecting the reading of very small lesions.
The rate of silent brain infarcts at study entry (27%) was slightly higher than that reported in the CSSCD study (21.8%)2 but lower than that reported for STOP (37%),18 which may be attributed to exclusion of patients with abnormal MRA or those who failed to convert to normal TCD in STOP II. The prevalence of silent infarcts in the STOP II patients with normal MRA but early vasculopathy, defined by abnormal TCD, is similar to what has been recently reported in young children with mostly normal TCD.4 These data suggest the need for early intervention to prevent the development of silent infarcts, as these lesions may not be asymptomatic as the terminology implies. White matter lesions are associated with impaired cognitive skills suggesting they can be nearly as damaging to cognitive function as overt stroke.20 In addition to cognitive effects, silent infarcts have a role in the decline of other functional performances, and this places individuals with higher-grade or multiple lesions at increased risk of developing disability.20 Although patients in this report had no evidence of gross cognitive disabilities, minor and more specific cognitive disability or psychologic disease was not evaluated and cannot be fully ruled out.
The mechanism through which transfusions prevent the development of silent infarcts in patients with SCD is unknown. For patients at high-risk of overt stroke, a proportional reduction in flow velocity with increased levels of total hemoglobin, increase in red-cell mass, and reduction in red-cell adhesion to endothelium is proposed.21,22 Reduction of intravascular hemolysis and the resulting free hemoglobin, which consumes nitric oxide, may increase the capacity for cerebral vasodilatation in response to ischemic stress.23 In the STOP trial, regular transfusions were associated with a significant reduction in plasma free hemoglobin levels,24 and lactate dehydrogenase levels significantly increased over the course of the STOP II study in transfusion-halted patients.19 In this study, data regarding markers of hemolysis were difficult to interpret and thus the beneficial effect of transfusions in this setting cannot not be explained by suppression of hemolysis.
The absolute silent infarct risk reduction with transfusions in our study is 19.4%, which is comparable with data from STOP (14.1%).18 Although our findings clearly demonstrate the benefit of transfusion therapy for the prevention of silent brain infarcts, they cannot be generalized to all patients with SCD. The lack of correlation between the development of abnormal TCD velocity and silent cerebral infarction in this study is in an agreement with previous reports. High velocities on TCD and silent infarcts reveal different aspects of the pathophysiology of central nervous system injury in SCD and are often discordant,25 although both predict a higher risk for stroke.5,26 Similar to MRA, TCD reveals large-vessel disease whereas the mechanism of silent infarction in SCD seems to be essentially microvascular.27 The benefit of transfusion in the prevention of silent cerebral infarction in children with SCD seems to depend on the patient's cerebrovascular status.28 Analysis of the STOP trial revealed that transfusion therapy lowers the risk for new silent infarcts in children with early cerebral vasculopathy as determined by abnormal TCD velocity.18 A more recent report revealed that children with SCD who had overt strokes and were receiving regular blood transfusion continue to experience a high rate of silent cerebral infarcts.28 The role of transfusion therapy in preventing silent infarcts in children with normal TCD velocities has been questioned.2 Our study reveals that at least in patients with abnormal TCD velocities who revert to normal with transfusions, discontinuation from the transfusion program will increase the risk of silent infarction. The Silent Cerebral Infarct Transfusion (SIT) trial will help expand on our findings as it is currently evaluating whether blood transfusion therapy will reduce further neurologic morbidity in children with silent cerebral infarcts, but with normal TCD velocities.29
Despite the benefits in preventing neurologic complications of SCD, several potential complications are associated with chronic transfusion therapy. Although kept to a minimum by careful screening and matching of blood products, the risks of transmission of viral infection and alloimmunization increase with recurrent transfusion. In addition, iron overload is an inevitable consequence of chronic transfusion therapy.30 Although earlier and more frequent introduction of blood transfusions will increase the rate of iron accumulation, effective methods of iron overload detection and chelation in children with SCD are now available,30-32 and the benefits of transfusion therapy may greatly outweigh the cost and inconvenience of iron chelation therapy. Exchange transfusions can also be used to avoid iron accumulation, or even to decrease iron burden.33 Alternates to transfusion, such as hydroxyurea therapy and stem cell transplantation, are also available. Stem cell transplantation has a role in the secondary prevention of strokes while preliminary results on hydroxyurea are unfavourable.34 However, the role of both modalities in the primary prevention of strokes is yet to be determined.
In conclusion, our study echoed primary results of the STOP II trial as it showed that discontinuing transfusion in children with SCD placed on stroke prophylaxis regimens is associated with the development of silent brain infarction. Together with the data from STOP,18 our findings suggest that transfusions are highly effective in preventing silent infarcts in patients with early cerebral vasculopathy (abnormal TCD but normal MRA). As silent infarcts are associated with significant morbidity in patients with SCD, and transfusions do not seem to prevent their development in patients with advanced vasculopathy,28 more studies are needed to define patients at risk and develop effective preventive strategies. This is especially important as TCD screening cannot be used to predict silent infarcts.11
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to the patients and their families for their contribution to this research. We would also like to thank the New England Research Institutes and all contributors to the STOP II trial as listed in the original manuscript, especially the Magnetic resonance reading panel: R. Zimmerman (chair), J. Bello, and F. Moser.
The original STOP II trial was supported by grants (U01 HL 052193 and U01 HL 052016) from the National Heart, Lung, and Blood Institute.
National Institutes of Health
Authorship
Contribution: M.R.A. and R.J.A. contributed to the study design; E.Y. and K.M.M. contributed to data preparation and analysis; and all the authors contributed to analysis, review, manuscript preparation and final approval for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of the principal investigators in the STOP II trial can be found in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Miguel R. Abboud, MD, Professor of Pediatrics, Hematology and Oncology, Children's Cancer Center of Lebanon, Chairman, Department of Pediatrics and Adolescent Medicine, American University of Beirut Medical Center; PO Box 11-0236; Beirut 1107 2020, Lebanon; e-mail: abboudm@aub.edu.lb.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal