Abstract
Notch signaling plays a central role in cell-fate determination, and its role in lateral inhibition in angiogenic sprouting is well established. However, the role of Notch signaling in lymphangiogenesis, the growth of lymphatic vessels, is poorly understood. Here we demonstrate Notch pathway activity in lymphatic endothelial cells (LECs), as well as induction of delta-like ligand 4 (Dll4) and Notch target genes on stimulation with VEGF or VEGF-C. Suppression of Notch signaling by a soluble form of Dll4 (Dll4-Fc) synergized with VEGF in inducing LEC sprouting in 3-dimensional (3D) fibrin gel assays. Expression of Dll4-Fc in adult mouse ears promoted lymphangiogenesis, which was augmented by coexpressing VEGF. Lymphangiogenesis triggered by Notch inhibition was suppressed by a monoclonal VEGFR-2 Ab as well as soluble VEGF and VEGF-C/VEGF-D ligand traps. LECs transduced with Dll4 preferentially adopted the tip cell position over nontransduced cells in 3D sprouting assays, suggesting an analogous role for Dll4/Notch in lymphatic and blood vessel sprouting. These results indicate that the Notch pathway controls lymphatic endothelial quiescence, and explain why LECs are poorly responsive to VEGF compared with VEGF-C. Understanding the role of the Notch pathway in lymphangiogenesis provides further insight for the therapeutic manipulation of the lymphatic vessels.
Introduction
The Notch pathway is a highly conserved signaling system that controls cell-fate determination. Recently, Notch has been shown to control endothelial cell (EC) proliferation, motility, filopodia formation, adhesion, and vessel stabilization.1,2 Among the Notch receptors, the endothelium expresses mainly Notch1 and Notch4, which are activated by Delta-like or Jagged family ligands presented in trans by the neighboring cells. This leads to cleavage of the receptor at the juxtamembrane domain by γ-secretase to release the Notch intracellular domain (NICD), which translocates to the nucleus to regulate downstream gene expression.1,2 Loss-of-function studies using inhibitors of Dll4 or Notch1, heterozygous Dll4 gene deletion, or inhibition of Notch1 cleavage with γ-secretase inhibitors all demonstrated excessive sprouting of blood vascular endothelial cells.1,2 Interestingly, Dll4 inhibitors produced excessive angiogenesis also in tumors, but the perfusion of the newly formed vessels was compromised and thus tumor growth was retarded.3-5
Lymphatic vessels are critical for the maintenance of tissue fluid balance, immune responses, and absorption of hydrophobic nutrients in the gut.6 Lymphangiogenesis, the growth of new lymphatic vessels, is an essential process during embryonic development.6,7 Although usually quiescent in adults, lymphatic vessels can sprout from preexisting vessels and anastomose to form new vessels in pathologic conditions, such as inflammation and tumor progression, where production of lymphangiogenic factors is induced. VEGF-C and VEGF-D, acting through VEGF receptor 3 (VEGFR-3), are key inducers of lymphangiogenesis.6 Loss of Vegfc leads to complete aplasia of the lymphatic vessels and embryonic lethality because of edema,8 whereas VEGF-D is dispensable for lymphatic development in mice.6,9
VEGF-C and VEGF-D are also capable of activating VEGFR-2 after proteolytic processing in the extracellular space.6 Although VEGFR-3 signals are sufficient for inducing lymphangiogenesis,10 VEGFR-2, the key receptor driving angiogenesis, is also expressed in the lymphatic endothelium and signaling via this receptor induces circumferential hyperplasia, but not sprouting of lymphatic vessels in vivo, as assessed by adenoviral expression of human or murine VEGF, or a VEGFR-2–specific ligand, VEGF-E, derived from the Orf virus.11-13
VEGF, the major ligand for VEGFR-2, is critical for angiogenesis,14,15 but its role in physiologic and pathologic lymphangiogenesis is not well understood. Heterozygous loss of Vegf or homozygous inactivation of Vegfr2 leads to death of mice at around embryonic day (E) 8.5 because of failure to form blood vessels.14-16 As the lymphatic vessels begin to develop considerably later, at around E10.5, the contribution of the VEGF/VEGFR-2 signaling pathway to lymphatic vessel development has not been addressed by direct gene-targeting studies.6 Overexpression of VEGF was shown to promote peritumoral lymphangiogenesis and metastasis to distal lymph nodes,17,18 but blocking VEGFR-2 in a prostate tumor model, although inhibiting tumor growth and angiogenesis, failed to suppress lymph node metastasis.19 Nevertheless, combinational silencing of both VEGF-C and VEGF showed synergistic benefit in blocking lymph node and lung metastases.20 However, it is noteworthy that VEGF can recruit VEGFR-1–positive macrophages that produce VEGF-C and VEGF-D,21,22 which makes it difficult to assess the direct role of VEGF in lymphangiogenesis.
VEGF has thus been implicated as a weak lymphangiogenic factor despite VEGFR-2 expression in the lymphatic endothelium. Although Dll4-Notch interactions play an important role in angiogenesis, it is not known whether similar mechanisms are at play during lymphatic vessel sprouting. For example, the mechanisms regulating tip-versus-stalk cell specification in lymphatic endothelial cells (LECs) have remained enigmatic. Here we show that suppression of Notch signaling, in synergy with VEGF, induces lymphangiogenesis both in vitro and in vivo. We also demonstrate that the Notch ligand Dll4 determines the tip/stalk fate during lymphangiogenesis in analogy to angiogenesis.
Methods
Production and purification of Dll4-Fc
293T cells were transfected with Dll4-Fc or HSA (Fugene6 Transfection Reagent; Roche Diagnostics), and cultured in serum-free medium 24 hours after transfection. The supernatant was collected on the following day, concentrated 10 times with Centrifugal Filter Units (membrane size: 10 kDa; Millipore), aliquoted and frozen. Dll4-Fc was purified from the supernatant by protein G chromatography (GE Healthcare) according to the manufacturer's instructions. Protein concentration was determined by the Pierce BCA Protein Assay Kit (Thermo Scientific).
Immunoprecipitation and Western blotting
Cells were lysed in RIPA buffer (150mM NaCl, 1% Nonidet P40 [NP40], 0.5% sodium deoxycholate, 0.1% SDS, 50mM Tris, 1 μg/mL aprotinin, 1 μg/mL leupeptin, 1mM NaF, 1mM NaVO4, 1mM PMSF). Cell lysates were separated by SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) membranes, and incubated with Abs directed against the cleaved Notch1 intracellular domain (cNICD; Cell Signaling Technology), VEGFR-2 (R&D Systems), VEGFR-3 (Millipore), phospho-Akt (Cell Signaling Technology), phospho-Erk1/2 (Cell Signaling Technology), phospho-tyrosine (Upstate Biotechnology), Prox-1 (R&D Systems), HSC70 (Santa Cruz Biotechnology), or β-actin (Cell Signaling Technology), followed by appropriate HRP-conjugated secondary Abs. For immunoprecipitation, cell lysates were incubated with VEGFR-2 Ab (R&D Systems) overnight and 1 hour with protein G Sepharose (GE Healthcare) at +4°C, and subjected to Western blot analysis.
Quantitative PCR analysis
LECs were treated, as indicated in the figure legends, in EC medium containing 0.5% FCS. Total RNA was isolated with the RNAeasy Mini Kit (Qiagen) and reverse-transcribed with the DyNAmo cDNA Synthesis Kit (Finnzymes). Quantitative PCR (qPCR) was performed in technical triplicates using the DyNAmo HS SYBR Green qPCR kit or the Probe qPCR kit (Finnzymes). Gene expression was normalized to β-actin or GAPDH. Relative fold changes were calculated by the formula, 2−ΔΔCt, where Ct stands for threshold cycles. Primer information can be found in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Three-dimensional bead sprouting assay
Cytodex 3 microcarrier beads (GE Healthcare) were coated with endothelial cells (mixed at 400 cells per bead) in Microvascular Endothelial Cell Growth Medium-2 MV (Lonza), and embedded in 2 mg/mL fibrin gels in 48-well plates by mixing 2 mg/mL fibrinogen (Calbiochem) in HBSS, 1 U/mL thrombin (Sigma-Aldrich), and 150 μg/mL aprotinin (Sigma-Aldrich). Endothelial Cell Growth Medium-2 (Lonza) containing WI-38 cells (11 000 per well) was added to each well in the presence of human VEGF-C (100 ng/mL), Dll4-Fc– or HSA-conditioned medium, Compound X (30mM),23 VEGFR-2 blocking Ab (7 μg/mL; Imclone), VEGFR1-Fc24 (7 μg/mL), VEGFR3-Fc,24 VEGFR-3 blocking Ab (3C5, 10 μg/mL; Imclone), human IgG (7 μg/mL, 10 μg/mL, or 20 μg/mL; Sigma-Aldrich), purified Dll4-Fc (20 μg/mL), or their indicated combinations. The cultures were maintained for 6-9 days by changing the medium every other day before fixation with 4% paraformaldehyde (PFA) for 1 hour at room temperature (RT). Bright field images were captured with Axiovert 200 (Zeiss) at 5× magnification and sprout lengths were measured with NIH ImageJ.
3D spheroid sprouting assay
Three thousand LECs were cultured in round-bottom 96-well plates (Nunc) precoated with 0.8% agarose for 1 day for spheroid formation. The spheroids were then collected and embedded in 20% Matrigel-containing (BD Biosciences) fibrin gels (3 mg/mL fibrinogen, 2 U/mL thrombin, and 200 μg/mL aprotinin), treated with hIgG (Sigma-Aldrich), Dll4-Fc, human VEGF165 (R&D Systems), VEGF-C, LY29200 (Calbiochem), ephrinB2 blocking peptide (see supplemental Methods for details) or their combinations in EC medium (PromoCell) containing 1% FCS at final concentrations indicated in the figure legends for 48 hours. The spheroids were then fixed in 4% PFA for 1 hour at room temperature (RT).
The tip cell competition assay was performed similarly, except that the LECs were transduced with vehicle or mDll4-ECTM-EGFP, prelabeled with Orange and Green CellTracker (Invitrogen), respectively, according to the manufacturer's protocol and mixed 1:1 for spheroid formation. A fraction of the mixed cells were plated on a coverslip and stained for mDll4 to evaluate the percentage of mDll4-ECTM-EGFP–transduced cells in the total population.
In vivo use of the viral vectors
The Provincial State Office of Southern Finland approved all animal experiments. Details of viral transduction experiments are found in supplemental Methods.
Immunofluorescent staining
Details of the whole-mount staining of ears, beads, and spheroids are found in supplemental Methods. For cleaved NICD staining, LECs cultured on coverslips were treated with Dll4-Fc or hIgG (10 μg/mL) in 0.5% FCS-containing medium overnight and fixed with 4% PFA for 5 minutes at RT. The cells were then stained with an Ab specifically targeting the cleaved NICD (Cell Signaling Technology) using the TSA system (PerkinElmer) to amplify the signal, according to the manufacturer's protocol, along with PECAM-1 (Dako) Ab, and mounted in mounting medium containing DAPI (Vector Laboratories).
Fluorescent microscopy
All the whole-mount stained samples were imaged using a confocal microscope (Zeiss LSM 510, air objectives: 10× with numerical aperture [NA] 0.5; oil objectives: 40× with NA 1.3) at RT with LSM AIM software. 3D projections were digitally reconstructed from Z-stacks. For measurement of VEGFR-3 and Prox1-positive vessel area per microscopic area, color images were converted to grayscale images using Adobe Photoshop and analyzed with ImageJ. Measurement of NICD and DAPI intensity was performed similarly. Brightness and contrast of the images were adjusted using Adobe Photoshop.
Quantitative analysis and statistics
Images for quantitation were coded with numbers and evaluated blindly to the treatments. Data were presented as mean ± SEM except for the tip cell-competition assay, where percentages were used. For comparison of means, 2-way ANOVA without interaction followed by the Holm-Sidak test was used. For comparison of proportions, the 2-tailed binomial test was used. Statistical analyses were carried out with SPSS 17.0 software or manually. Statistical significance is indicated in the figures by asterisks: *P < .05, **P < .01, and ***P < .001.
More information on the methods and materials can be found in supplemental Methods.
Results
Characterization of Notch pathway components, the soluble inhibitor of Notch, and its effects on the downstream Notch targets in LECs
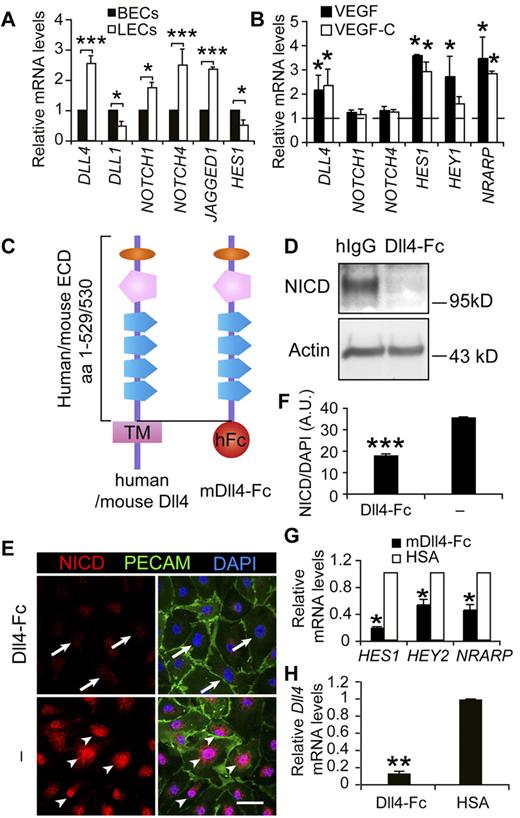
To study the role of Notch signaling in LECs, we first examined the expression levels of several main components of the pathway. qPCR analysis of cultured human blood vascular endothelial cells (BECs) and LECs indicated that Notch receptors, ligands, and downstream targets were expressed at comparable levels in these 2 cell types (Figure 1A), suggesting an active role for Notch signaling in the LECs. To understand whether Notch signaling is regulated by VEGF or VEGF-C signaling, which are involved in LEC activation,6 we analyzed the expression of DLL4, NOTCH1, and NOTCH4 in LECs in response to VEGF and VEGF-C. Whereas NOTCH1 and NOTCH4 expression were essentially unchanged, DLL4 as well as the downstream Notch target genes HES1, HEY1, and NRARP were up-regulated by VEGF or VEGF-C treatment (Figure 1B). Dll4 was also induced in the lymphatic endothelium by VEGF or VEGF-C expression in vivo, although weak expression was also detected in resting vessels (supplemental Figure 1).
Notch pathway components, the soluble inhibitor of Notch and its effects on the downstream Notch targets in LECs. (A) Comparison of mRNA expression levels of Notch signaling molecules between cultured human BECs and LECs. (B) qRT-PCR analysis of DLL4, NOTCH1, NOTCH4, HES1, HEY1, and NRARP in LECs treated with VEGF (100 ng/mL) or VEGF-C (100 ng/mL) for 1 hour. The dashed line indicates the nontreated control levels, which are taken as 1. (C) Diagram of the domain structures of human and mouse full-length Dll4 and mDll4-Fc. The latter comprises the extracellular domain (ECD) of mDll4 and the Fc domain of human IgG. (D) Western blot analysis of cleaved N1ICD in LECs treated with Dll4-Fc or hIgG (10 μg/mL) overnight. β-actin served as a loading control. (E) Immunofluorescent staining for cleaved N1ICD (red), PECAM-1 (green), and DAPI (blue) of LECs treated with Dll4-Fc (10 μg/mL) overnight or untreated. Arrows indicate diminished N1ICD staining in the nuclei and arrowheads the active N1ICD. Scale bar, 50 μm. (F) Quantification of N1ICD in the nuclei in panel E. (G) qRT-PCR analysis showing inhibition of Notch target gene expression by Dll4-Fc in LECs 24 hours after treatment. (H) qPCR analysis of DLL4 expression in LECs treated with Dll4-Fc– or HSA-conditioned medium for 24 hours. Data represent means ± SEM of at least 3 independent experiments; *P < .05; **P < .01; ***P < .001.
Notch pathway components, the soluble inhibitor of Notch and its effects on the downstream Notch targets in LECs. (A) Comparison of mRNA expression levels of Notch signaling molecules between cultured human BECs and LECs. (B) qRT-PCR analysis of DLL4, NOTCH1, NOTCH4, HES1, HEY1, and NRARP in LECs treated with VEGF (100 ng/mL) or VEGF-C (100 ng/mL) for 1 hour. The dashed line indicates the nontreated control levels, which are taken as 1. (C) Diagram of the domain structures of human and mouse full-length Dll4 and mDll4-Fc. The latter comprises the extracellular domain (ECD) of mDll4 and the Fc domain of human IgG. (D) Western blot analysis of cleaved N1ICD in LECs treated with Dll4-Fc or hIgG (10 μg/mL) overnight. β-actin served as a loading control. (E) Immunofluorescent staining for cleaved N1ICD (red), PECAM-1 (green), and DAPI (blue) of LECs treated with Dll4-Fc (10 μg/mL) overnight or untreated. Arrows indicate diminished N1ICD staining in the nuclei and arrowheads the active N1ICD. Scale bar, 50 μm. (F) Quantification of N1ICD in the nuclei in panel E. (G) qRT-PCR analysis showing inhibition of Notch target gene expression by Dll4-Fc in LECs 24 hours after treatment. (H) qPCR analysis of DLL4 expression in LECs treated with Dll4-Fc– or HSA-conditioned medium for 24 hours. Data represent means ± SEM of at least 3 independent experiments; *P < .05; **P < .01; ***P < .001.
We produced a soluble, competitive Notch inhibitor, comprising the extracellular domain of mouse Dll4 and the Fc domain of human IgG, designated as Dll4-Fc (Figure 1C). Dll4-Fc effectively inhibited the cleavage and nuclear localization of NICD, using an Ab specifically recognizing the cleaved form (Figure 1D-F), and the expression of Notch target genes, including HES1, HEY2, and NRARP, in LECs (Figure 1G). Interestingly, inhibition of Notch signaling with Dll4-Fc also suppressed the expression of DLL4 in LECs (Figure 1H), indicating that in addition to blocking the ligand-receptor interaction, Dll4-Fc further inhibited Notch signaling at the gene expression level of the pathway by down-regulating DLL4.
Blocking Notch promotes LEC sprouting in vitro
To study whether endogenous Notch signals are involved in inhibiting LEC sprouting, as has been demonstrated for BECs, we applied Dll4-Fc to LEC-coated beads embedded in 3D fibrin gels that were covered by WI-38 human fibroblasts in the presence or absence of VEGF-C (Figure 2A). Conditioned medium containing Dll4-Fc or purified protein stimulated robust sprouting of the LECs, whereas beads stimulated with the control medium or hIgG did not form sprouts (Figure 2A-B). Compound X, a γ-secretase inhibitor that inhibits Notch signaling,23 also induced LEC sprouting (Figure 2C). Taken together, these results indicate an important role for Notch signaling in the regulation of lymphatic sprouting.
Blockade of Notch signaling results in LEC sprouting. (A) Immunofluorescent PECAM-1 staining of LEC-coated microbeads subjected to Dll4-Fc– or HSA-conditioned medium with or without VEGF-C (100 ng/mL). (B) Quantification of the cumulative sprout lengths and number per bead in the experiment of panel A. (C) PECAM-1 staining of LEC-coated beads treated with the γ-secretase inhibitor Compound X (X, 30mM) or DMSO (vehicle). Scale bars, 100 μm. Data summarized from 2 independent experiments with conditioned media and a third independent experiment with purified Dll4-Fc (20 μg/mL), with similar results (n = 8-15 per group in each experiment) in panel B and 3 independent experiments in panel C. Error bars, SEM; ***P < .001; N.S., not significant.
Blockade of Notch signaling results in LEC sprouting. (A) Immunofluorescent PECAM-1 staining of LEC-coated microbeads subjected to Dll4-Fc– or HSA-conditioned medium with or without VEGF-C (100 ng/mL). (B) Quantification of the cumulative sprout lengths and number per bead in the experiment of panel A. (C) PECAM-1 staining of LEC-coated beads treated with the γ-secretase inhibitor Compound X (X, 30mM) or DMSO (vehicle). Scale bars, 100 μm. Data summarized from 2 independent experiments with conditioned media and a third independent experiment with purified Dll4-Fc (20 μg/mL), with similar results (n = 8-15 per group in each experiment) in panel B and 3 independent experiments in panel C. Error bars, SEM; ***P < .001; N.S., not significant.
Notch blockade enhances lymphatic sprouting induced by VEGF
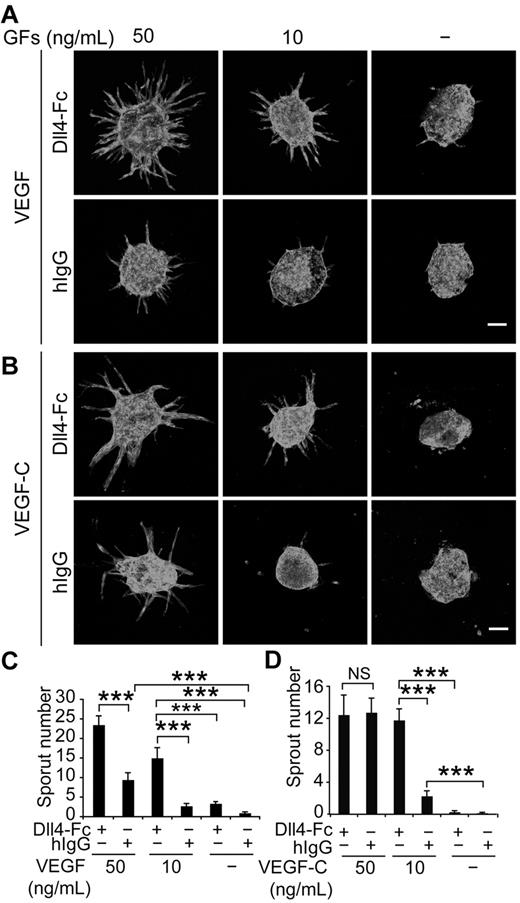
Because VEGF is an integral component of the medium in the bead-sprouting assay, and the cocultured fibroblasts also produce various growth factors, we resorted to an EC spheroid-sprouting assay, which does not require coculture of fibroblasts, and allows the titration of VEGF and VEGF-C. As shown in Figure 3A and C, inhibition of Notch by Dll4-Fc synergized with VEGF in inducing lymphatic sprouting in a dose-dependent fashion, whereas VEGF alone was less effective. Although Dll4-Fc also increased the sprouting induced by low concentration of VEGF-C (10 ng/mL), this effect was lost when the concentration of VEGF-C increased to 50 ng/mL (Figure 3B,D). Taken together, these data suggest that Notch signaling inhibits VEGF-induced lymphatic sprouting, whereas VEGF-C can override this restriction at sufficient doses.
Notch inhibition and VEGF stimulation synergize in lymphatic sprouting in vitro. (A) LEC spheroids were treated with Dll4-Fc (20 μg/mL), VEGF (10 or 50 ng/mL), or their combination as indicated and stained for PECAM-1. Note the synergistic induction of LEC sprouting by Dll4-Fc and VEGF in a dose-dependent manner, as quantified in panel C. GFs indicates growth factors. (B) Similar titration of VEGF-C in the presence or absence of Dll4-Fc (20 μg/mL). (D) Quantification of the sprout number in the experiment shown in panel B. Scale bar, 100 μm. Data represent means ± SEM from 2-3 independent experiments with n = 6-13 per group per experiment; *P < .05; **P < .01; ***P < .001.
Notch inhibition and VEGF stimulation synergize in lymphatic sprouting in vitro. (A) LEC spheroids were treated with Dll4-Fc (20 μg/mL), VEGF (10 or 50 ng/mL), or their combination as indicated and stained for PECAM-1. Note the synergistic induction of LEC sprouting by Dll4-Fc and VEGF in a dose-dependent manner, as quantified in panel C. GFs indicates growth factors. (B) Similar titration of VEGF-C in the presence or absence of Dll4-Fc (20 μg/mL). (D) Quantification of the sprout number in the experiment shown in panel B. Scale bar, 100 μm. Data represent means ± SEM from 2-3 independent experiments with n = 6-13 per group per experiment; *P < .05; **P < .01; ***P < .001.
Dll4-Fc potentiates VEGF-induced lymphangiogenesis in vivo
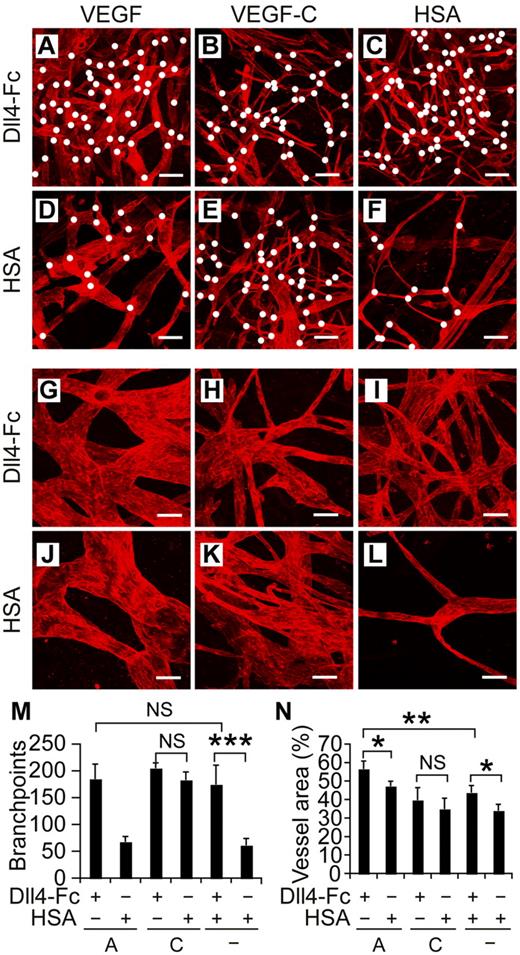
To assess the concomitant effects of Notch inhibition and VEGF or VEGF-C stimulation in vivo, we injected recombinant adeno-associated virus (rAAVs) encoding Dll4-Fc, VEGF, VEGF-C, or HSA intradermally into mouse ears (Figure 4A-L). As in the bead sprouting assay, Dll4-Fc alone (Figure 4C,I) induced lymphangiogenesis as quantified by both number of branch points and vessel area (Figure 4M-N), whereas no lymphangiogenesis was observed in ears transduced with the HSA control virus (Figure 4F,L). The addition of rAAV-VEGF to Dll4-Fc increased the vessel area compared with Dll4-Fc alone, although we did not observe increased branching in the combination experiment (Figure 4A-N). In line with our previous results,11 expression of human VEGF alone did not induce sprouting or branching, but only circumferential hyperplasia of the lymphatic vessels (Figure 4J,L-N). As in the bead sprouting assay and the spheroid sprouting assay with VEGF-C (Figures 2A-B and 3C-D), Dll4-Fc did not significantly increase the VEGF-C induced lymphatic vessel area or number of branch points (Figure 4B,E,M-N).
Notch inhibition and VEGF cooperate to induce lymphangiogenesis in vivo. (A-F) Lymphatic vessels of mouse ears were visualized by VEGFR-3 whole-mount staining (red) 2 weeks after transduction with rAAVs encoding the indicated factors. White dots indicate branchpoints. (G-L) Higher magnifications of panels A through F. Note the increased branching and circumferential hyperplasia of lymphatic vessels in the VEGF and Dll4-Fc combination shown in panels A and G. Scale bars indicate 200 μm (A-F) and 50 μm (G-L). Quantification of the branchpoints (M), as indicated by the white dots in panels A through F, and vessel area (N) per microscopic field. A indicates VEGF; C, VEGF-C, and HSA, human serum albumin. Shown are means ± SEM summarized from 2 independent experiments with n = 4-6 per group per experiment; *P < .05; **P < .01; ***P < .001.
Notch inhibition and VEGF cooperate to induce lymphangiogenesis in vivo. (A-F) Lymphatic vessels of mouse ears were visualized by VEGFR-3 whole-mount staining (red) 2 weeks after transduction with rAAVs encoding the indicated factors. White dots indicate branchpoints. (G-L) Higher magnifications of panels A through F. Note the increased branching and circumferential hyperplasia of lymphatic vessels in the VEGF and Dll4-Fc combination shown in panels A and G. Scale bars indicate 200 μm (A-F) and 50 μm (G-L). Quantification of the branchpoints (M), as indicated by the white dots in panels A through F, and vessel area (N) per microscopic field. A indicates VEGF; C, VEGF-C, and HSA, human serum albumin. Shown are means ± SEM summarized from 2 independent experiments with n = 4-6 per group per experiment; *P < .05; **P < .01; ***P < .001.
Role of VEGFR-2 and VEGFR-3 signaling in lymphangiogenesis induced by Notch inhibition
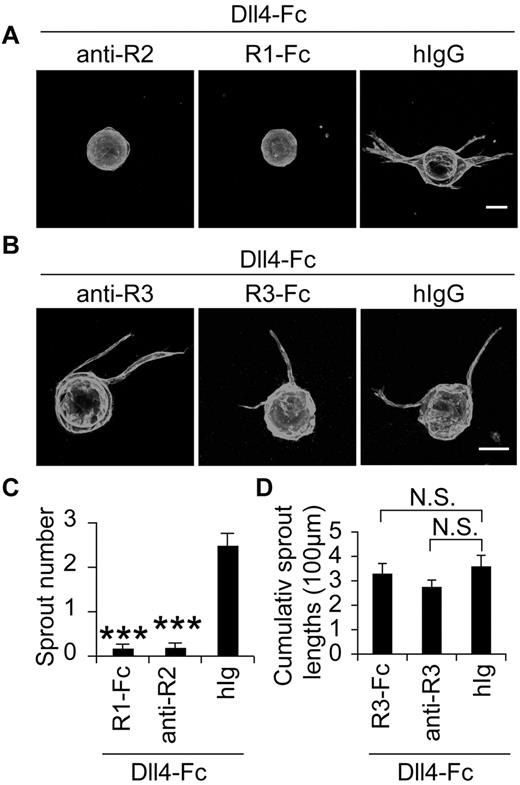
As LECs express both VEGFR-3 and VEGFR-2, which can both stimulate lymphangiogenesis,6 we wondered whether the sprouting induced by Notch blockade is dependent on the activation of these receptors. In the bead-sprouting assay, where VEGF was contained in the medium, either a blocking Ab against VEGFR-2 or a soluble VEGFR-1 (VEGFR1-Fc), serving as a VEGF trap, almost completely blocked the effect of Dll4-Fc (Figure 5A,C), indicating that VEGF/VEGFR2 signals control the lymphatic sprouting induced by Notch inhibition. However, blockade of VEGFR-3 signaling by a blocking Ab against VEGFR-3 or VEGFR3-Fc, a VEGFR-3 trap, did not significantly suppress LEC sprouting in response to Dll4-Fc (Figure 5B,D).
Involvement of VEGFR-2 and VEGFR-3 in the lymphatic sprouting induced by Notch inhibition in vitro. (A-B) LEC-coated beads were treated with a blocking Ab against VEGFR-2 (5 μg/mL) or VEGFR-3 (10 μg/mL), a soluble VEGFR-1-Fc (5 μg/mL) or VEGFR3-Fc (5 μg/mL) or control hIgG, in the presence of Dll4-Fc (20 μg/mL) and stained for PECAM-1. Scale bars, 100 μm. (C-D) Quantification of the sprouting in panels A and B. anti-R2 indicates anti–VEGFR-2; anti-R3, anti-VEGFR-3; R1-Fc, VEGFR-1-Fc; and R3-Fc, VEGFR-3-Fc. Results represent means ± SEM summarized from 2 independent experiments with n = 6-12 per group per experiment; ***P < .001 compared with the last bar in panel C.
Involvement of VEGFR-2 and VEGFR-3 in the lymphatic sprouting induced by Notch inhibition in vitro. (A-B) LEC-coated beads were treated with a blocking Ab against VEGFR-2 (5 μg/mL) or VEGFR-3 (10 μg/mL), a soluble VEGFR-1-Fc (5 μg/mL) or VEGFR3-Fc (5 μg/mL) or control hIgG, in the presence of Dll4-Fc (20 μg/mL) and stained for PECAM-1. Scale bars, 100 μm. (C-D) Quantification of the sprouting in panels A and B. anti-R2 indicates anti–VEGFR-2; anti-R3, anti-VEGFR-3; R1-Fc, VEGFR-1-Fc; and R3-Fc, VEGFR-3-Fc. Results represent means ± SEM summarized from 2 independent experiments with n = 6-12 per group per experiment; ***P < .001 compared with the last bar in panel C.
In vivo, the lymphangiogenesis induced by Dll4-Fc in vivo was suppressed by VEGFR1-Fc and VEGFR-3(domains 1-3)-Fc (also called VEGF-C/D trap), a competitive inhibitor of VEGFR-3, but not by an inactive VEGFR-3(domains 4-7)-Fc, which does not have ligand-binding activity and served as a control (Figure 6), indicating that VEGFR-2 and VEGFR-3 signaling regulate the lymphangiogenesis induced by Notch inhibition in vivo.
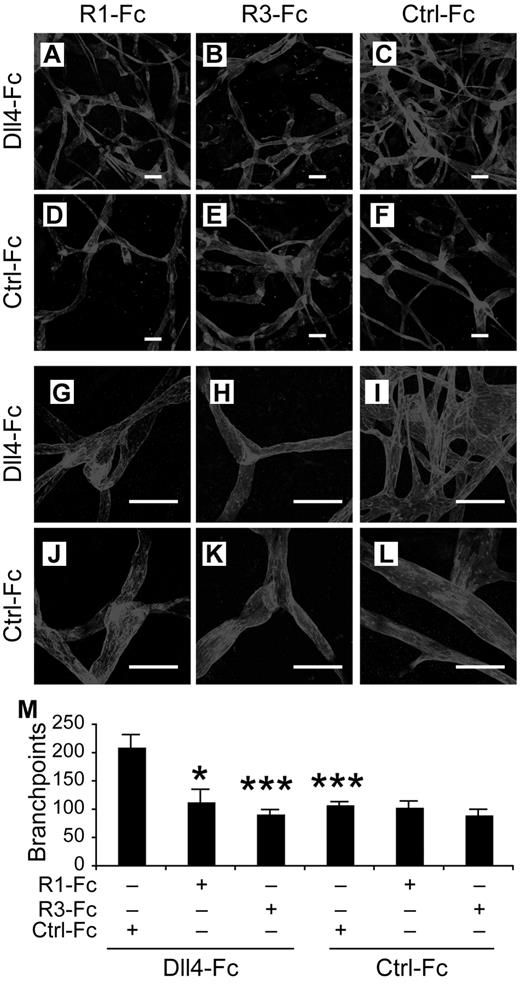
Requirements for VEGFR-2 and VEGFR-3 in Notch inhibition-induced lymphatic sprouting in vivo. (A-F) Lymphatic vessels stained by VEGFR-3 2 weeks after transduction of mouse ears with the indicated rAAVs. (G-M) Close-up views of panels A through F. (N) Quantification of the ear lymphatic vessel branchpoints in mice transduced with the indicated rAAVs. R1-Fc indicates VEGFR-1-Fc; and R3-Fc, VEGFR-3(domain1-3)-Fc. Ctrl-Fc indicates VEGFR-3(domain 4-7)-Fc, used as a negative control. Scale bars, 100 μm. Results represent means ± SEM summarized from 2 independent experiments with n = 4-8 per group per experiment; *P < .05; ***P < .001 compared with the first bar in panel N.
Requirements for VEGFR-2 and VEGFR-3 in Notch inhibition-induced lymphatic sprouting in vivo. (A-F) Lymphatic vessels stained by VEGFR-3 2 weeks after transduction of mouse ears with the indicated rAAVs. (G-M) Close-up views of panels A through F. (N) Quantification of the ear lymphatic vessel branchpoints in mice transduced with the indicated rAAVs. R1-Fc indicates VEGFR-1-Fc; and R3-Fc, VEGFR-3(domain1-3)-Fc. Ctrl-Fc indicates VEGFR-3(domain 4-7)-Fc, used as a negative control. Scale bars, 100 μm. Results represent means ± SEM summarized from 2 independent experiments with n = 4-8 per group per experiment; *P < .05; ***P < .001 compared with the first bar in panel N.
Regulation of VEGFR-2, VEGFR-3, and ephrinB2 signaling by Notch signaling in LECs
To dissect the mechanism of how Notch signaling regulates lymphatic sprouting, we determined the expression levels of VEGFR-2 and VEGFR-3. Neither VEGFR-2 nor VEGFR-3 expression at the mRNA or protein level was significantly altered by exposure to Dll4-Fc in LECs cultured in a 2-dimensional monolayer (supplemental Figure 2A-C). Furthermore, the phosphorylation of VEGFR-2 stimulated by VEGF was not affected by Dll4-Fc treatment (supplemental Figure 2D). The serine-threonine kinase Akt is known to regulate EC migration and angiogenesis,25 and Akt is phosphorylated on VEGF and VEGF-C stimulation in LECs.26 We therefore asked whether Akt was important in lymphatic sprouting induced by VEGF and Notch inhibition. Blocking Akt activity with LY292002, an inhibitor of PI3K, efficiently abrogated LEC sprouting induced by combined VEGF and Dll4-Fc treatment (supplemental Figure 3A-B). However, treatment with Dll4-Fc did not enhance VEGF-induced Akt phosphorylation (supplemental Figure 3C), suggesting that while Akt is important for VEGF-induced LEC sprouting, it is not directly regulated by the Notch pathway. Similarly, phosphorylation of the MAPK Erk1/2 was not altered by Dll4-Fc in the presence or absence of VEGF (supplemental Figure 3C).
In BECs, ephrinB2 has been shown to be a target of Notch signaling and involved in EC sprouting,27 we therefore asked whether ephrinB2 was also regulated by Notch in LECs and accounted for the LEC sprouting induced by Notch inhibition. Indeed, ephrinB2 expression was suppressed by Dll4-Fc, both at the basal level and in the presence of VEGF (supplemental Figure 4A). However, blocking the interaction between ephrinB2 and EphB4 using a blocking peptide did not enhance LEC sprouting induced by VEGF or VEGF-C, but rather inhibited the sprouting (supplemental Figure 4B-C).
Inhibition of Notch signaling does not affect Prox1 expression in adult mouse tissues or in vitro
Prox1 is a master regulator of lymphatic development, whose deficiency results in failure of lymphatic vessel formation.28 We studied Prox1 expression in our in vivo model, as it was recently suggested that overactivation of the Notch signaling pathway can suppress Prox1 expression in cultured LECs.29 We found that inhibiting Notch by Dll4-Fc did not affect Prox1 expression in adult lymphatic vessels (supplemental Figure 5A-B), nor did it alter Prox1 expression in cultured LECs (supplemental Figure 5C).
Notch signaling regulates tip-stalk specification in LECs
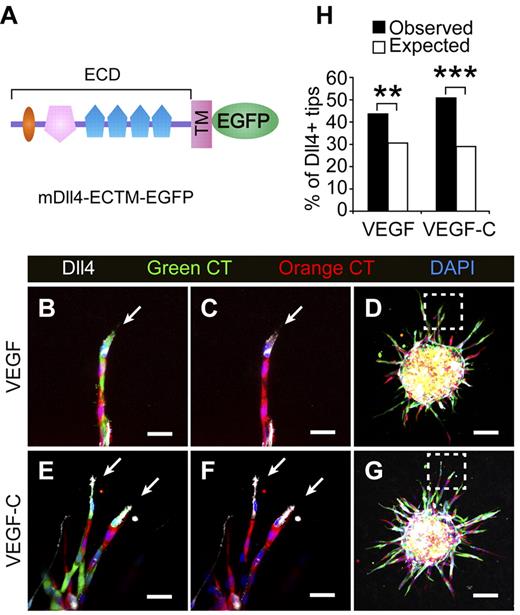
In vitro and in vivo studies have shown that Notch is cell autonomously required for stalk cell specification in BECs, and that cells with a low degree of Notch signaling and abundant Dll4 expression become selected as tip cells.30,31 We therefore hypothesized that the LECs expressing more Dll4 win the competition for the tips. To address this question, a retrovirus expressing Dll4 extracellular and transmembrane domains fused to EGFP (DLL4-ECTM-EGFP, Figure 7A) was constructed and used to transduce cultured LECs. The cells were labeled with green CellTracker and mixed with empty retrovirus-transduced LECs that were labeled with orange CellTracker to form mosaic spheroids. A fraction of the mixed cells were plated on coverslips and stained for mDll4 (30% positive in the total population; Figure 7H). When stimulated with VEGF or VEGF-C, the percentage of Dll4-positive cells in the tip cell position was significantly higher (increased to 43% and 50%, respectively) than expected (30%; Figure 7B-H), indicating that the tip cell position is determined by Dll4/Notch signals during lymphangiogenesis in analogy to BEC sprouting.
Lymphatic endothelial cells expressing Dll4 show an advantage in competition for the tip cell position. (A) Schematic representation of the mouse Dll4-EGFP fusion protein. (B-G) LECs transduced with mDll4-ECTM-EGFP or empty retrovirus were prelabeled with Green (green) or Orange (red) CellTracker (CT), respectively, and mixed in a 1:1 ratio to form the spheroids, which were treated with VEGF (100 ng/mL) or VEGF-C (100 ng/mL) for 48 hours. The spheroids were subsequently stained for mDll4 (white) and DAPI (blue). The arrows indicate Dll4+ tip cells. (D,G) Low-power magnification of the spheroids in panels B,C,E and F (□). Scale bars, 25 μm in panels B,C,E and F and 100 μm in panels D and G. (H) The percentage of mDll4-positive tip cells (white cells in panels A-D) out of the total tip cells was quantified and plotted against their expected values. The figure summarizes data from 2-3 independent experiments with a total of n = 122 for VEGF and n = 229 for VEGF-C treatments. A 2-tailed binomial test was used for statistical analysis. **P < .01; ***P < .001.
Lymphatic endothelial cells expressing Dll4 show an advantage in competition for the tip cell position. (A) Schematic representation of the mouse Dll4-EGFP fusion protein. (B-G) LECs transduced with mDll4-ECTM-EGFP or empty retrovirus were prelabeled with Green (green) or Orange (red) CellTracker (CT), respectively, and mixed in a 1:1 ratio to form the spheroids, which were treated with VEGF (100 ng/mL) or VEGF-C (100 ng/mL) for 48 hours. The spheroids were subsequently stained for mDll4 (white) and DAPI (blue). The arrows indicate Dll4+ tip cells. (D,G) Low-power magnification of the spheroids in panels B,C,E and F (□). Scale bars, 25 μm in panels B,C,E and F and 100 μm in panels D and G. (H) The percentage of mDll4-positive tip cells (white cells in panels A-D) out of the total tip cells was quantified and plotted against their expected values. The figure summarizes data from 2-3 independent experiments with a total of n = 122 for VEGF and n = 229 for VEGF-C treatments. A 2-tailed binomial test was used for statistical analysis. **P < .01; ***P < .001.
Discussion
In this study we demonstrate that Notch signaling molecules are expressed at comparable levels in LECs and BECs. Interestingly, inhibition of Notch worked in synergy with VEGF to induce lymphatic sprouting both in vitro and in vivo. The induction of lymphangiogenesis in response to Notch pathway inhibition seems to depend on VEGFR-2 and VEGFR-3 signaling. Furthermore, forced expression of Dll4 in LECs promoted adoption of the tip cell position. These results suggest that the Notch pathway negatively regulates lymphatic sprouting and directs stalk cell specification in LECs.
Role of Notch signaling in LECs
In cultured LECs, the key components of the Notch pathway were expressed at levels comparable with, or even higher than those in BECs, indicating that Notch signaling is important for controlling lymphangiogenesis. Notch1 and Notch4, the 2 Notch receptors expressed in the vascular endothelium,1,2 have been shown to be expressed in lymphatic vessels in normal and tumor tissues,32 indicating that Notch signaling is active in the lymphatic endothelium in both physiologic and pathologic conditions.
Several papers showed that the Notch pathway was important for blood vessel remodeling and ischemia-induced angiogenesis, whereas blockade of Notch signaling resulted in impaired migration, proliferation, and survival of BECs and impaired the recovery of blood flow in response to ischemia.33,34 However, the principal role of endothelial Notch signaling appears to rather lie in the regulation of blood vessel quiescence and lateral inhibition of new sprout formation during angiogenesis: studies from mouse retina models, tumor models, and in vitro 3D sprouting assays have established that Notch signaling serves as a negative regulator of VEGF-induced angiogenesis, and that inhibition of the Notch pathway leads to excessive sprouting of blood vessels and nonproductive angiogenesis.1
The role of Notch signaling in LECs is less well understood. A recent study reported that genetic targeting of Notch impaired LEC migration during embryonic development in a zebrafish model.35 However, results from our mammalian model using adult mice indicated that inhibition of Notch signaling rather induced lymphangiogenesis. This was further supported by 3D in vitro sprouting assays using human LECs subjected to a Notch inhibitor, consistent with the negative role of Notch in regulating the sprouting of the blood vessels.1 The discrepancy between the study by Geudens et al35 and our current work may be explained by the dual function of Notch in inducing cell differentiation and suppressing cell growth, which may be differently regulated in embryonic development and in adults. It is conceivable that, in the embryos, Notch signaling is important for the formation, remodeling, or maturation of the lymphatic vasculature, whereas in adults its role is shifted to maintenance of the quiescence of the established vessels. This certainly seems to be the case for blood vessels in adults, as chronic inhibition of Dll4/Notch signaling was recently shown to lead to spontaneous hyperproliferation of BECs and the formation of hemangiomas,36 and Notch1 haploinsufficiency resulted in vascular tumors.37 In addition, the differences between the endothelial Notch receptors in zebrafish (Notch1b and Notch6) and in mammals (Notch1 and Notch4) may also contribute to the discrepancy.
Notch inhibition results in nonproductive blood vessels that are poorly perfused.1 Whether the sprouting lymphatic vessels induced by Notch inhibition are functional remains enigmatic. We and others have previously shown that the lymphatic vessels are poorly functional during sprouting and growth, but they gradually stabilize and become functional within a few months after growth factor stimulation.38,39 The lymphatic vessels induced by Dll4-Fc likely undergo a similar course of events.
Interaction between Notch signaling and the VEGF family
Although VEGF potently promotes angiogenesis and sprouting of BECs in vivo, it does not stimulate LEC sprouting, but rather promotes circumferential enlargement of the lymphatic vessels accompanied by LEC proliferation.11 Here we show that VEGF up-regulates Dll4 expression in LECs, which activates Notch to suppress the lymphatic sprouting in response to VEGF. Our gene expression analysis indicated that LECs have a higher baseline expression of several Notch pathway components compared with BECs. Thus the weaker effects of VEGF on LECs compared with BECs could be explained by the higher activity of Notch in the LECs at baseline. The VEGF-C/VEGFR-3 pathway also activated Notch signaling in LECs, which seemed to negatively regulate VEGF-C–induced lymphatic sprouting when VEGF-C levels were low, but once VEGFR-3 was fully activated by VEGF-C, it was able to overcome the restriction imposed by Notch and potently induced LEC sprouting. However, the grip on VEGF by Notch was tighter, as Notch blockade could still enhance VEGF-induced sprouting when VEGF was used at high concentrations.
In vivo, combinational treatment with VEGF and Dll4-Fc induced both circumferential enlargement and sprouting of lymphatic vessels, whereas Dll4-Fc did not increase VEGF-C–induced sprouting, consistent with the in vitro bead-sprouting assay. Thus, the lymphatic sprouting induced by VEGF-C/VEGFR-3 is essentially unrestrained by Notch, whereas the lymphatic sprouting activity of VEGF/VEGFR-2 is rather weak because of the tight restriction imposed by Notch signaling. This is consistent with the fact that the VEGFR-2 and VEGFR-3 signals in the ECs, although generally overlapping, show some differences. For example in angiogenesis, VEGFR-3 activation seems to potently promote EC migration rather than proliferation.40,41 It should also be noted that in the LECs, VEGFR-3 is expressed much more abundantly than VEGFR-2,42 which may explain why Notch does not restrict VEGFR-3–induced sprouting.
The lymphatic sprouting after Notch inhibition seemed to depend on the VEGF/VEGFR-2 pathway, as Dll4-Fc by itself did not induce sprouting in the spheroid assay of pure LECs, but potentiated VEGF-induced sprouting to a similar extent as was achieved by VEGF-C alone. The dependence on VEGF/VEGFR-2 signals was further supported by the fact that both a VEGFR-2 blocking Ab and VEGF trapping by VEGFR-1-Fc ablated the Dll4-Fc–induced sprouts both in vivo and in vitro. This is not surprising, given that the increased endothelial filopodia in the developing retinas of Dll4+/− mice were also suppressed by an Ab targeting VEGFR-2 or by soluble VEGFR-1.43 The requirement for VEGFR-3 is more complicated. Whereas VEGFR-3 is dispensable for Dll4-Fc–induced LEC sprouting in vitro, blocking VEGFR-3 signaling suppressed Dll4-Fc–induced lymphangiogenesis in vivo. The importance of VEGFR-3 signaling for lymphangiogenesis in vivo is evidenced by the fact that activation of VEGFR-2 could not rescue the lymphatic regression caused by prolonged systemic VEGFR3-Fc expression.11 Importantly, Notch inhibition did not enhance LEC sprouting induced by VEGF-C in any of the assays. Thus, it is possible that in vivo, a low level of VEGF-C or VEGFR-3 activity is required to prime LECs to become responsive to VEGF, for example, by controlling a critical step in sprout initiation.44 However in the downstream, lymphatic sprouting induced by a high dose of VEGF, but not of VEGF-C, is restricted by Notch signaling, as inhibition of Notch signaling enhanced sprouting in response to VEGF, but not to VEGF-C. In our in vivo model of adult mouse ears, inhibition of Notch alone induced lymphangiogenesis. It therefore seems that blocking Notch was able to sensitize the lymphatic endothelium to endogenous growth factor signals emanating from the surrounding microenvironment. Similar endothelial hyperreactivity was observed in certain blood vascular beds of rats and cynomolgus monkeys that were exposed to Notch inhibitors for prolonged periods of time.36
Mechanism of lymphatic sprouting induced by Notch inhibition
The exact mechanism of how Notch inhibits sprouting has not been fully elucidated. In vivo and in vitro studies show that Notch activation down-regulates VEGFR-2 expression in the BECs, thus making them less responsive to VEGF.1 Although cultured BECs up-regulate VEGFR-3 on NICD overexpression,32 inhibition of Notch in vivo resulted in widespread VEGFR-3 expression and sprouting of blood vessels.40 In the LECs, a recent study showed that endothelial overexpression of the Notch1 intracellular domain (N1ICD) up-regulated VEGFR-2 expression, whereas the Notch targets Hey1 and Hey2, but not Hes1 or NICD, could down-regulate VEGFR-3.29 Another study using N1ICD overexpression did not report changes in the percentage of LECs positive for VEGFR-3.32 We observed neither significant alteration in the expression of VEGFR-2 or VEGFR-3, nor in VEGF-induced VEGFR-2 phosphorylation when blocking Notch signaling in LECs. Actually, whereas activation of Notch signaling suppressed VEGFR-2 expression, Notch blockade by γ-secretase inhibitor did not alter VEGFR-2 levels in cultured BECs.45 Similarly, in vivo, Notch inhibition failed to increase VEGFR-2 expression in the retinal vessels.46 Thus, although forced activation of Notch signaling may tilt the balance of gene expression to one side, in the loss-of-function condition the balance can be maintained by alternative pathways. Taken together, these results imply that Notch signaling probably does not restrict VEGF-induced lymphangiogenesis at the VEGF receptor level, but rather acts on a downstream signaling cascade that would otherwise contribute to the sprouting.
EphrinB2 expression was reduced by Notch signaling targeting, as previously shown in BECs,27 but ephrinB2 blockade did not reproduce the stimulatory effects of Dll4-Fc, and rather inhibited the LEC sprouting induced by VEGF and VEGF-C, suggesting that Notch regulates ephrinB2 in a negative feedback loop to control LEC sprouting. In fact, the effects of the ephrinB2 blocking peptide are most likely because of modulation of VEGFR-2 and VEGFR-3 signaling.47,48 Thus, in addition to the interplay between VEGF and Notch signaling, a third signal may be also integrated in the signaling network to determine the final outcome of vessel quiescence/sprouting. Other Notch targets that account for LEC sprouting induced by Notch inhibition remain to be explored.
Role of Notch in the LEC differentiation
Mosaic analysis of BECs in mice and zebrafish demonstrated that Notch controls tip-stalk specification in growing sprouts, as evidenced by the dominant tip cell adoption of the Notch-deficient cells and, conversely, exclusion of NICD overexpressing cells from the tip position.31,49 According to this “tug-of-war” concept, neighboring ECs compete for the tip position through contact-dependent Dll4-Notch signaling.1 Here, we demonstrate that similar cell-fate control by Notch also holds true in the LECs: the cells with forced Dll4 expression preferentially adopt the tip cell position, presumably through a similar mechanism as in BECs.
Regulation on the transcription factor Prox1, the master gene specifying lymphatic cell fate,28 by Notch signaling has been studied in early embryonic development and in vitro. Endothelial deletion of Rbpj, a transcriptional coactivator acting in concert with NICD to activate Notch target genes, did not interfere with Prox1 expression or lymphatic development when analyzed at E10.0.50 Our in vivo data suggest that Notch signaling does not influence Prox1 expression in adult lymphatic vessels, although this conclusion needs to be confirmed by investigation of later embryonic and postnatal stages of development. In cultured LECs, overexpression of NICD suppressed Prox1 expression,29 whereas in our experiments Notch suppression did not alter Prox1 levels.
Taken together, we established a negative role of Notch signaling in controlling lymphatic sprouting induced by VEGF, and provided an explanation for the weak lymphangiogenic activity of VEGF despite ample levels of VEGFR-2 expression in the LECs. This finding should have considerable biological significance, as it provides a further mechanistic explanation for the remarkable specificity of VEGF for stimulating angiogenesis. Furthermore, our results caution the possible future use of Notch pathway inhibitors in the treatment of cancer, as they may sensitize tumor-associated lymphatic vessels to VEGF, promote tumor lymphangiogenesis, and drive metastatic spreading of the malignancy.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Michael Jeltsch for the VEGF-C protein, Denis Tvorogov and Ralf Adams for helpful discussions, Tapio Tainola for expert technical assistance, the Molecular Imaging Unit for microscopy support, and the staff at the Biomedicum Helsinki and the Haartman Institute Animal Facilities for excellent animal husbandry.
This work was supported by grants from the Finnish Academy, the Finnish Cancer Research Organizations, the European Community's Seventh Framework Program FP7/2007-2011 grant 201279, the Association for International Cancer Research, and the Helsinki University Research Fund.
Authorship
Contribution: W.Z. and T.T. designed and performed the research, analyzed the results, and wrote the paper; M.Y. and A.A. constructed AAV vectors and produced the viruses; T.H. performed serologic evaluation of AAV transduction; S.K. generated the Dll4 plasmids; T.K. generated VEGFR3(D4-7)-Fc; K.L. contributed to the spheroid-sprouting assay; S.Y.-H. provided the adenoviruses; and K.A. designed the research, interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no compet-ing financial interests.
Correspondence: Kari Alitalo, Molecular/Cancer Biology Laboratory, Research Programs Unit, Institute for Molecular Medicine Finland and Helsinki University Hospital Biomedicum Helsinki, PO Box 63 (Haartmaninkatu 8), Mail Code 00014, Helsinki, Finland; e-mail: Kari.Alitalo@Helsinki.Fi.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal