Abstract
Nonmyeloablative allogeneic transplantation (NMAT) infrequently cures active chemoresistant, bulky, or aggressive B-cell lymphoma (B-cell non-Hodgkin lymphoma [B-NHL]). We hypothesized that 90Y-ibritumomab tiuxetan–based NMAT would facilitate early cytoreduction in such patients promoting improved long-term disease control by the allogeneic graft. Forty high-risk B-NHL patients with persistent disease received 0.4 mCi/kg (maximum, 32 mCi/kg) 90Y-ibritumomab tiuxetan, fludarabine, and 2 Gy total body irradiation and matched-related (15) or unrelated (25) transplantation. Baseline features included: median age, 58 years (range, 29-69 years); median prior regimens, 6 (range, 3-12); chemosensitive disease, 6 (15%); bulk > 5 cm, 17 (range, 5.2-18.6 cm, 43%); diffuse large B-cell lymphoma, 14 (35%); and comorbidity score > zero, 34 (85%). Early responses were observed in 24 (60%, 14 complete remission/complete remission unconfirmed, 10 partial response) patients, including 17 of 29 (59%) with chemotherapy-resistant disease and 10 (59%) with bulk > 5 cm. The estimated 30-month survival, progression-free survival, and nonrelapse mortality were 54.1%, 31.1%, and 15.9%, respectively. Early response, baseline platelet counts over 25 000/μL, indolent histology, and related donors were associated with improved survival. The addition of 90Y-ibritumomab tiuxetan to NMAT is safe and yields early responses and prolonged disease control in some of the highest-risk B-NHL patients. This trial was registered at www.clinicaltrials.gov as #NCT00119392.
Introduction
Patients with heavily pretreated B-cell non-Hodgkin lymphoma (B-NHL) and comorbidities or advanced age have few effective treatment options and little chance for cure. Nonmyeloablative allogeneic transplantation (NMAT) has the potential to eradicate disease in such patients with reduced or delayed nonrelapse mortality (NRM).1-5 Data from a variety of centers suggest that long-term progression-free survival (PFS) rates range from 30% to 80% depending on the histology, patient, and disease factors.5-7 The reduced intensity of the conditioning regimen has also allowed patients with advanced age or comorbidities to benefit from this approach. However, the lack of an intensive conditioning regimen enhances the risk of early relapse as disease control is nearly exclusively mediated by the graft-versus-lymphoma (GVL) effect, which can take several months to manifest.8 Not surprisingly, data indicate that patients with bulky, chemoresistant disease or aggressive histology not in complete remission (CR) often have disease progression before the establishment of effective tumor control by the graft.3,4,9,10 Unfortunately, simply intensifying the conditioning regimen for such patients would be expected to, at best, improve tumor control at the expense of higher rates of NRM.11
Radioimmunotherapy (RIT) has emerged as one of the most effective single agents in relapsed B-NHL, with responses after one administration seen in 50% to 80% patients with relapsed follicular lymphoma.12-15 Data also exist suggesting that this modality has efficacy in more aggressive histologies, such as diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL).16-18 Advantages of RIT over more traditional chemotherapeutic regimens include the limited nonhematologic toxicity and the potential for circumventing the chemotherapy resistance seen in many patients with advanced lymphoma.
We hypothesized that the addition of RIT to a NMAT regimen would deliver safe and effective cytoreduction and thus provide additional time for the allogeneic graft to establish a robust GVL effect. Furthermore, we anticipated that the allogeneic graft would abrogate the expected hematologic toxicity after RIT. Patients in need of this strategy generally may have disease that is too aggressive for a standard NMAT approach and too many comorbidities or advanced age for a fully myeloablative conditioning regimen. Herein, we present the results of a prospective phase 2 trial evaluating a conditioning regimen of 90Y-ibritumomab tiuxetan to augment antitumor activity with fludarabine and low-dose total body irradiation (TBI) to ensure engraftment before matched related or unrelated allogeneic hematopoietic cell transplantation in such high-risk patients with persistent relapsed or refractory lymphoid malignancies.
Methods
Patient and donor selection
Patients were eligible if they were 18 years of age or older, had a histologically confirmed diagnosis of B-cell lymphoma or chronic lymphocytic leukemia (CLL) expressing the CD20 antigen, had failed at least one prior regimen, and had evidence of persistent disease. Patients were excluded if they had major organ dysfunction, had received systemic antilymphoma therapy within 30 days of the 90Y dose, had active central nervous system tumor involvement, had an ECOG performance status more than 2, were unwilling to use contraceptive techniques, had active infection, or were in CR. Patients who had received a prior murine antibody were required to have no evidence of human anti–mouse antibody formation. Patients with an altered biodistribution of 111In-ibritumomab tiuxetan were also excluded.
Related donors required matching by intermediate resolution molecular typing for HLA-A, -B, -C, -DRB1, and -DQB1 according to Fred Hutchinson Cancer Research Center Standard Practice Guidelines and by high resolution typing for -DRB1.19 Unrelated donors required allele matching for HLA-A, -B, -C, and -DRB1 by high resolution typing and DQB1 by intermediate resolution typing. A single allele disparity for HLA-A, -B, or -C was allowed for both related and unrelated donors.
Treatment plan and follow-up
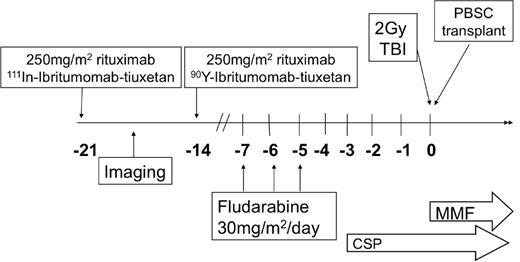
All patients underwent baseline evaluation with physical examination, computed tomography, and bone marrow studies. On day −21 before allogeneic transplantation, 250 mg/m2 of rituximab was administered before an imaging dose of 111In-ibritumomab tiuxetan, as previously published.12 A minimum of one γ-camera image was obtained to confirm the expected biodistribution of the radiotracer. 111In-ibritumomab tiuxetan γ-camera images for all patients were independently reviewed by 2 radiologists (J.R., M.C.M.) for qualitative biodistribution and tumor visualization. On day −14, 250 mg/m2 of rituximab was administered before the therapy dose of 0.4 mCi/kg of 90Y-ibritumomab tiuxetan, with a maximum dose of 32 mCi. There was no dose reduction for preexisting cytopenias, and all therapy was administered as an outpatient. To optimize the potential synergy between RIT and nucleoside analogs, fludarabine was administered at 30 mg/m2 daily on days −7, −6, and −5.9,20 A total of 2 Gy TBI was delivered on day 0. Graft-versus-host disease (GVHD) prophylaxis included cyclosporine and mycophenolate mofetil as previously published.1 Cyclosporine was continued until day 56 for patients with related donors and then tapered off by day 180, and was continued until day 100 for those with unrelated donors and tapered over 11 weeks. Mycophenolate mofetil was stopped on day 27 for patients with related donors, whereas those with unrelated donors continued at full dose until day 40 and tapered off by day 96. No patients received donor lymphocyte infusion for persistent disease or low chimerism. The overall treatment schema is illustrated in Figure 1.
Treatment schema. CSP indicates cyclosporine; and MMF, mycophenolate mofetil.
Patients were restaged with computed tomography scans and bone marrow evaluations at 1, 3, and 6 months after transplantation and then annually thereafter. Donor chimerism studies were performed at 1 and 3 months and then annually. GVHD evaluations followed standard institutional practice.
Endpoints and statistical considerations
The primary objective of the study was to demonstrate that the inclusion of 90Y-ibritumomab tiuxetan as part of a NMAT regimen resulted in a 100-day NRM of < 20%. Secondary endpoints included overall and complete response rates, overall survival (OS), and PFS. Radiographic responses were evaluated by independent radiographic review using standard criteria.21 Chemosensitive disease was defined as achieving a CR or partial response (PR) to the cytoreductive regimen immediately before transplant conditioning. DLBCL was considered an aggressive lymphoma, whereas CLL/small lymphocytic lymphoma (SLL), follicular lymphoma (FL), hairy cell leukemia (HCL), and marginal zone lymphoma (MZL) were considered indolent. MCL was evaluated separately. Adverse events were scored using the National Cancer Institute Common Toxicity Criteria, Version 3.0. Cumulative incidence curves were used to estimate the probabilities of time-to-event outcomes, and all curves were truncated when fewer than 3 patients were at risk for an event. Disease progression was treated as a competing risk in the analysis of NRM. The statistical significance of differences in event rates was evaluated with the proportional hazards regression model. Disease response at 3 months was treated as a time-dependent covariate in the model predicting OS from time of transplantation. Reported P values are 2-sided and based on the Wald statistic. This trial was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board, and all patients provided written informed consent before participating in this trial, in accordance with the Declaration of Helsinki. This trial was registered at clinicaltrials.gov (#NCT00119392).
Results
Patient characteristics
Forty-five patients with advanced B-NHL were consented to this study between October 2004 and February 2009. Five patients did not complete therapy and were not evaluable, including 2 who experienced explosive progression of disease and clinical deterioration in performance status between consent and therapy, 2 who requested to be withdrawn from the study before transplantation, and one who was found to have an altered 111In-ibritumomab tiuxetan biodistribution. Four of 5 died of disease progression a median of 82 days (range, 60-202 days) from consent. The remaining 40 patients were fully evaluable for the primary endpoint. Characteristics included (Table 1): median age, 58 years (range, 29-69 years); median number of prior regimens, 6 (range, 3-12 prior regimens); median comorbidity score, 3 (range, 0-10),22 disease bulk > 5 cm, 17 (43%, range, 5.2-18.6 cm); and chemosensitive disease, 6 (15%). None of the patients was in CR. Seventeen (43%) patients had received a prior transplant (16 autologous, 1 allogeneic). Of the 29 patients with documented chemoresistant disease, the most recent regimen before transplantation was ifosphamide/carboplatin/etoposide, with or without rituximab (R; 6), cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP) with or without R (6), dexamethasone/high-dose cytarabine/cisplatin (3), bortezomib with or without R (3, 2 had previously failed to respond to R-ifosphamide/carboplatin/etoposide, and one failed to respond to R-etoposide, methylprednisolone, high-dose cytarabine, cisplatin [ESHAP]), R-ESHAP (2), R-hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone (R-hyperCVAD, 1), R-cyclophosphamide, etoposide, procarbazine, prednisone (1), high-dose cyclophosphamide, etoposide, and dexamethasone (1), high-dose methotrexate/cytarabine (1), IFN-α (1, previously refractory to cladribine), vincristine-prednisone (1, previously refractory to fludarabine, cyclophosphamide, and R, FCR), dose-intensive cyclophosphamide, etoposide, and cisplatin (1), cyclophosphamide/vincristine/prednisone (CVP, 1, previously refractory to R-CHOP), FCR. (1) Histologies included: DLBCL (14, including 7 that had transformed from indolent disease), CLL/SLL (10), MCL (8), FL (6), HCL (1), and MZL (1). Twenty-one patients (53%) had an international prognostic index score of ≥ 3 when measured before transplantation. Furthermore, 10 (25%) patients had > 25% bone marrow cellularity involvement (range of marrow involvement, 40%-95%), 19 (48%) had platelets < 100 000/μL, and 9 (23%) had neutrophils < 1000/μL at baseline.
Baseline patient characteristics (n = 40)
| Characteristic . | Value . |
|---|---|
| Age, y, median (range) | 58 (29-69) |
| Female, no. (%) | 13 (33) |
| Stage III/IV, no. (%) | 40 (100) |
| Prior regimens, median (range) | 6 (3-12) |
| Prior transplant, no. (%) | 17 (43) |
| Chemosensitive, no. (%) | 6 (15) |
| CR, no. (%) | 0 (0) |
| Bulk > 5 cm, no. (%) | 17 (43) |
| > 25% marrow cellularity involved with NHL, no. (%) | 10 (25) |
| Baseline platelet count < 25 000/μL, no. (%) | 7 (18) |
| Comorbidity score ≥ 1, no. (%) | 34 (85) |
| International Prognostic Index at transplantation ≥ 3, no. (%) | 21 (53) |
| Histology, no. (%) | |
| Indolent | 18 (45) |
| De novo diffuse large B-cell | 7 (18) |
| Transformed diffuse large B-cell | 7 (18) |
| Mantle cell | 8 (20) |
| Donor, no. (%) | |
| Matched-related | 15 (38) |
| Matched-unrelated | 25 (62) |
| Characteristic . | Value . |
|---|---|
| Age, y, median (range) | 58 (29-69) |
| Female, no. (%) | 13 (33) |
| Stage III/IV, no. (%) | 40 (100) |
| Prior regimens, median (range) | 6 (3-12) |
| Prior transplant, no. (%) | 17 (43) |
| Chemosensitive, no. (%) | 6 (15) |
| CR, no. (%) | 0 (0) |
| Bulk > 5 cm, no. (%) | 17 (43) |
| > 25% marrow cellularity involved with NHL, no. (%) | 10 (25) |
| Baseline platelet count < 25 000/μL, no. (%) | 7 (18) |
| Comorbidity score ≥ 1, no. (%) | 34 (85) |
| International Prognostic Index at transplantation ≥ 3, no. (%) | 21 (53) |
| Histology, no. (%) | |
| Indolent | 18 (45) |
| De novo diffuse large B-cell | 7 (18) |
| Transformed diffuse large B-cell | 7 (18) |
| Mantle cell | 8 (20) |
| Donor, no. (%) | |
| Matched-related | 15 (38) |
| Matched-unrelated | 25 (62) |
Additional details of the CLL/SLL patients include 8 of 10 with documented fludarabine refractory disease with 6 experiencing no response or progressive disease while receiving fludarabine-containing combination regimens, one progressed 1 month after FCR and was taken directly to transplantation, and the final patient progressed 9 months after FR but failed to respond to PCR or CHOP and was taken to transplantation. One patient with unknown fludarabine sensitivity achieved a 50% reduction in adenopathy and lymphocytosis to FCR but was found to have central nervous system involvement. This patient was given intensive intrathecal chemotherapy, craniospinal radiotherapy, and enrolled on this protocol within 4 months of fludarabine. The final CLL/SLL patient achieved a 24-month remission after initial FR followed by rituximab maintenance, but at relapse failed to respond to R-CVP, R-CHOP, R-ESHAP, or R-bortezomib, and was taken to transplant. Six of 10 CLL/SLL patients had abnormal bone marrow cytogenetics before initiating conditioning with 4 of 6 demonstrating multiple complex cytogenetic abnormalities, and 2 with trisomy 12.
Therapy delivered
The median 90Y dose was 30.9 mCi (range, 21.7-32.6 mCi). All 40 patients received the planned doses of fludarabine and TBI. The median number of days between 90Y-ibritumomab tiuxetan and allogeneic stem cell infusion was 14 (range, 13-22 days). Patients received transplants from 15 matched related and 25 matched unrelated donors.
Response, progression, and survival
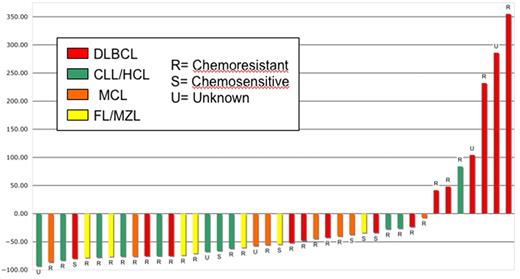
Early objective responses at 1 and 3 months were observed in 19 (48%, 3 CR/complete remission unconfirmed [CRu], 16 PR) and 24 (60%, 14 CR/CRu, 10 PR) patients, respectively, including 17 (59%) objective responses in the 29 patients with known chemoresistant disease and 10 (59%) of 17 with bulk more than 5 cm. Three-month responses were seen in 5 (38%, 5 CR) DLBCL, 9 (90%, 4 CR, 5 PR) CLL/SLL, 4 (50%, 2 CR, 2 PR) MCL, and 5 (83%, 3 CR, 2 PR) FL patients. The MZL and HCL patient achieved a PR and stable disease, respectively, by month 3. There was no significant difference in response between patients with DLBCL transformed from FL (3 CR) and those with de novo DLBCL (2 CR). In total, 33 (83%) patients experienced reduction in the measurable disease burden by day 84. Early response by histology and chemosensitivity is quantified in Figure 2. Figure 3 illustrates an example of an early response observed in a patient with highly chemoresistant, bulky CLL/SLL.
Waterfall plot of day 84 response after 90Y-ibritumomab tiuxetan-based NMAT by histology and chemoresistance. HCL indicates hairy cell leukemia. For the 4 patients who died before day 84, the most recent response data before death were used.
Waterfall plot of day 84 response after 90Y-ibritumomab tiuxetan-based NMAT by histology and chemoresistance. HCL indicates hairy cell leukemia. For the 4 patients who died before day 84, the most recent response data before death were used.
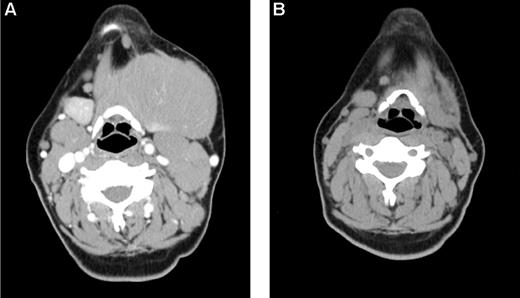
Early response in bulky, chemoresistant lymphoma. A 33-year-old woman with CLL/SLL had a partial response lasting 10 months after initial therapy with 6 cycles of fludarabine-R and then experienced progressive disease while receiving pentostatin-cyclophosphamide-R, and continued progressive disease while receiving CHOP. (A) Baseline image reveals a dominant 12 cm left submandibular mass. She was treated with 32 mCi of 90Y-ibritumomab tiuxetan, fludarabine, 2 Gy TBI, and a matched unrelated allogeneic transplant and achieved a PR by after transplantation day 28 (B) and complete remission by day 84. The patient remains alive and disease-free 3 years after transplantation.
Early response in bulky, chemoresistant lymphoma. A 33-year-old woman with CLL/SLL had a partial response lasting 10 months after initial therapy with 6 cycles of fludarabine-R and then experienced progressive disease while receiving pentostatin-cyclophosphamide-R, and continued progressive disease while receiving CHOP. (A) Baseline image reveals a dominant 12 cm left submandibular mass. She was treated with 32 mCi of 90Y-ibritumomab tiuxetan, fludarabine, 2 Gy TBI, and a matched unrelated allogeneic transplant and achieved a PR by after transplantation day 28 (B) and complete remission by day 84. The patient remains alive and disease-free 3 years after transplantation.
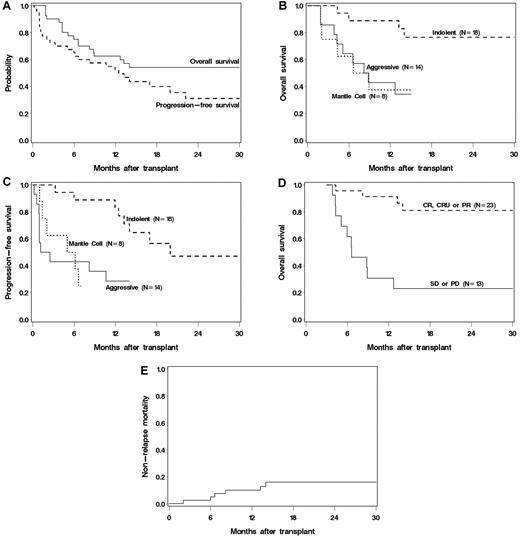
At a median follow up of 1.7 years, 19 patients remain alive and 14 alive and progression free. The 2-year estimated OS and PFS were 54% (95% confidence interval [CI], 37%-68%) and 31% (95% CI, 16%-48%), respectively (Figure 4A). The OS and PFS at 1.3 years of the 17 patients with prior transplantation (16 auto, 1 allo) was 50% (95% CI, 24%-62%), and 38% (95% CI, 14%-61%), respectively. Six of 11 with DLBCL relapsed and 8 died, none of 4 patients with indolent histologies relapsed but 1 died, and 1 of 2 MCL patients survived relapsed and the other died without relapse. Univariate analysis indicated superior OS and PFS in patients with indolent histology compared with those with aggressive or MCL (P < .01 for indolent vs others, Figure 4B-C). The estimated 6-month PFS in patients with indolent, MCL, and DLBCL were 89%, 50%, and 43%, respectively. Age, prior number of therapies, comorbidity score, chemosensitivity, and tumor bulk were not associated with OS. However, day 84 response (CR or PR) was strongly associated with a 5-fold improvement in OS (P < .001). Among patients who survived to day 84, those who responded had an estimated 91% 9-month OS compared with 31% in those that did not (Figure 4D). The best long-term disease control occurred in patients achieving early CRs. Relapse after the 3-month response assessment occurred in 3 of 7 with stable disease (43%), 5 of 10 with PR (50%), but only 2 of 13 (15%) with a CR/CRu at 3 months. Conversely, of the 10 patients with a PR at day 84, 2 converted to CR (CLL, FL) and 4 remained progression-free (MCL, CLL, FL, and MZL) by the 1-year evaluation, although one patient later relapsed. For the 7 patients with stable disease at day 84, none had achieved a CR by 1 year, but 2 remained progression-free (CLL, FL). None of the patients with progressive disease by day 84 survived to 1 year.
(A) OS and PFS of all treated patients. (B) OS by histology. (C) PFS by histology. (D) Landmark analysis of OS based on response at 3 months after transplantation. (E) Cumulative incidence of NRM.
(A) OS and PFS of all treated patients. (B) OS by histology. (C) PFS by histology. (D) Landmark analysis of OS based on response at 3 months after transplantation. (E) Cumulative incidence of NRM.
Multivariable analysis identified indolent histology (P < .001), baseline platelet count more than or equal to 25 000/μL (P = .002), and matched related donors (P = .04) as associated with improved OS (Table 2). In an additional model containing only patients who survived to day 84 after transplantation (n = 36), disease response remained a significant predictor of OS after adjusting for histology, baseline platelets, and donor type (P = .01). Likewise, indolent histology (P < .001), disease bulk ≤ 5 cm (P = .01), and platelet counts ≥ 25 000/μL (P = .01) were independently associated with improved PFS (Table 2).
Multivariable analysis
| . | HR (95% CI) . | P . |
|---|---|---|
| Factors associated with overall mortality | ||
| Histology group | ||
| Indolent | 1.0 | |
| Aggressive or mantle cell | 19.3 (3.9-94.5) | < .001 |
| Baseline platelets < 25 000/μL | ||
| No | 1.0 | |
| Yes | 11.0 (2.4-50.9) | .002 |
| Donor type | ||
| Matched-related | 1.0 | |
| Matched-unrelated | 3.6 (1.1-12.2) | .04 |
| Factors associated with progression or mortality | ||
| Histology group | ||
| Indolent | 1.0 | |
| Aggressive or mantle cell | 14.6 (4.0-53.7) | < .001 |
| Maximal disease bulk > 5 cm | ||
| No | 1.0 | |
| Yes | 3.6 (1.3-9.9) | .01 |
| Baseline platelets < 25 000/μL | ||
| No | 1.0 | |
| Yes | 5.1 (1.4-18.1) | .01 |
| . | HR (95% CI) . | P . |
|---|---|---|
| Factors associated with overall mortality | ||
| Histology group | ||
| Indolent | 1.0 | |
| Aggressive or mantle cell | 19.3 (3.9-94.5) | < .001 |
| Baseline platelets < 25 000/μL | ||
| No | 1.0 | |
| Yes | 11.0 (2.4-50.9) | .002 |
| Donor type | ||
| Matched-related | 1.0 | |
| Matched-unrelated | 3.6 (1.1-12.2) | .04 |
| Factors associated with progression or mortality | ||
| Histology group | ||
| Indolent | 1.0 | |
| Aggressive or mantle cell | 14.6 (4.0-53.7) | < .001 |
| Maximal disease bulk > 5 cm | ||
| No | 1.0 | |
| Yes | 3.6 (1.3-9.9) | .01 |
| Baseline platelets < 25 000/μL | ||
| No | 1.0 | |
| Yes | 5.1 (1.4-18.1) | .01 |
HR indicates hazard ratio.
Engraftment, graft-versus-host disease, and NRM
The addition of 90Y-ibritumomab tiuxetan to the conditioning regimen was well tolerated, although expected myelosuppression was observed. The median number of days after transplantation to a neutrophil > 500/μL was 17 (range, 0-34 days) and platelets > 50 000/μL was 11 days (range, 0-147 days). The neutrophil and platelet nadirs were 50/μL and 12 000/μL and occurred a median of 12 and 8 days after transplantation, respectively. The median duration of neutropenia < 500/μL was 11 days and thrombocytopenia < 50 000/μL was 8 days.
All patients experienced sustained engraftment, and there was no graft rejection. The median CD3 and CD33 peripheral blood donor chimerism levels at day 28 after transplantation were 96% and 100%, respectively. There was one (2.5%) non–relapse-related death before day 100 (primary endpoint) because of sepsis and multiorgan failure. The estimated cumulative NRM at 30 months was 16% (95% CI, 4%-28%) with late nonrelapse deaths related to infection/GVHD (n = 5) and bronchiolitis obliterans organizing pneumonia (n = 1; Figure 4E). Only baseline thrombocytopenia < 25 000/μL was associated with a higher rate of NRM (hazard ratio for death = 5.0; 95% CI, 1.0-24.9; P = .05). Acute GVHD developed in 31 (78%) patients before day 100, 4 of whom had grade 3 and none had grade 4. Chronic extensive GVHD was documented in 8 (20%) patients.
111In-ibritumomab tiuxetan γ-camera imaging
Thirteen of 40 (33%) patients had imageable tumor sites after 111In-ibritumomab tiuxetan, with all occurring in patients with at least 2 cm of disease bulk. There was no correlation of response with the ability to visualize disease sites when taken as a whole (61% overall response rate in imageable vs 59% overall response rate in nonimageable) or when evaluated within each histology group. However, 53% percent of patients with 5 cm or larger masses could be imaged compared with 17% in those with < 5 cm of disease (P = .04). The median bulk of visualizable disease was 6.6 cm compared with 3.6 cm for those that were nonimageable (P = .05). For the CLL patients, the marrow was imageable in 6 of 10, including 5 of 6 with > 5% marrow involvement. Interestingly, in the one CLL patient with heavy (50%) marrow involvement that was not imageable, CD20 was noted to be absent as measured by flow cytometry, yet identified intracellularly by immunohistochemistry (L26), potentially because of blocking of surface CD20 by prior R.
Discussion
This first large prospective phase 2 trial of a RIT-based allogeneic conditioning for patients with active residual B-NHL indicates that the addition of 90Y-ibritumomab tiuxetan to a standard NMAT regimen of fludarabine and low-dose TBI is safe and feasible with a 100-day NRM rate of 2.5% and a 2-year NRM of 16%. Importantly, this strategy was able to induce objective early remissions in the majority of these high-risk patients, none of whom was in CR, including those with chemoresistant and bulky disease. It is also of note that responses with standard-dose RIT were observed in histologies, such as DLBCL and CLL/SLL, where data using standard dose anti-CD20 RIT are limited. Together, this strategy was able to provide long-term remissions in nearly one-third of these patients who were in general not considered candidates for either standard myeloablative or nonmyeloablative transplantation because of patient and disease factors.
Our results also highlight one of the tenets in NMAT for lymphoma, the importance of early disease control in yielding long-term survival and remissions. Many series of NMAT have shown inferior outcomes in patients with chemoresistant, bulky, or rapidly progressive disease before transplantation.3,4,9,10 These characteristics were mirrored by the majority of patients in our study who were not able to gain adequate pretransplantation disease control. With the use of RIT, we were able to induce early responses in most patients, which were, like pretransplantation remission status, strongly associated with improved long-term survival. These data are particularly notable in the subset of DLBCL patients where there are no published data on the use of RIT-based NMAT and the impact of the GVL effect is less demonstrable.3,10 To date at a median follow up of 1.1 years (range, 0.7-3.7 years), none of the 5 DLBCL patients who achieved an objective response at 3 months has experienced disease progression. This observation supports the role of GVL for long-term disease control because all patients had active disease before transplantation and long-term remissions in this histology would be unlikely because of the conditioning regimen alone. However, it is impossible to discern which element of this regimen made the greatest contribution to prolonged remissions in DLBCL as the only patients not in early CR that were observed to experience delayed disease control between 3 and 12 months after transplantation were those with histologies where the GVL effect has been best described (indolent B-NHL and CLL). Nevertheless, these data suggest that the ability to gain early post-transplantation disease control in patients not responding to traditional chemoimmunotherapy regimens may provide a platform for long-term immune-mediated disease-free survival.
Many investigators have hypothesized that imaging data available after the 111In-ibritumomab tiuxetan infusion could be used to predict which patients might benefit most from the therapeutic infusion of 90Y-ibritumomab tiuxetan. As reflected by our findings, a single time point image of tumor uptake is not likely sufficient to predict those who may experience maximal benefit. This result is not necessarily unexpected. Although bulky disease was more readily imaged, prior data suggest that these patients are also more likely to have inferior biodistributions with reduced absorbed dose per gram of tissue.23 Bulk within certain groups may also reflect those with the most refractory disease and least likely to achieve an early response. For example, none of the 6 DLBCL or MCL patients with tumor masses more than 5 cm responded regardless of visualization of 111In within tumor sites. Properly addressing the question of tumor absorbed dose and response would require serial quantitative γ-camera imaging and tumor biopsies adjusted for histology.
Other series have also demonstrated encouraging outcomes after NMAT for lymphoma; however, comparing our data with these publications is problematic because of significant differences in baseline features. Such differences are also the case with the German experience using a similar regimen that was performed concurrently with our trial.24 Notably, this study, which delivered the fludarabine later in the conditioning regimen (days −4 to −2), excluded patients with aggressive NHL or those with progressive disease before transplantation. Our study, which delivered the fludarabine earlier in the conditioning regimen to take advantage of the potential synergy with RIT, was specifically designed for patients who were not candidates for standard NMAT.9,20 For example, our patients had more prior therapies, were less chemosensitive, had more bulky disease, had fewer pretransplant CRs, and had a greater fraction of patients with Hematopoietic Stem Cell Transplant Comorbidity Index scores 3 or higher compared with prior series (Table 3).1-5,24-26 For this reason, the patients and results from our trial could be considered a unique population, complementing the NMAT data in more favorable scenarios. Despite these adverse patient and disease features, the majority of patients in our study experienced early responses and nearly one-third were estimated to be alive and progression-free 2 years from transplantation. However, additional follow up is required to define the long-term curative potential of this regimen.
Baseline characteristics and outcomes of selected series of NMAT for lymphoma/CLL
| Reference . | n . | Age, y (median) . | Histology (no.) . | Regimen . | Chemosensitive, % . | CR at transplantation, % . | Prior treatment, no. . | Bulk > 5 cm, % . | HSCT-CI ≥ 3, % . | OS, % (y) . | PFS, % (y) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 47 | 53 | FL | FCR | 100 | 38 | 3 | NR | NR | 85 (5) | 83 (5) |
| 6 | 35 | 58 | MCL | PFA/FCR | 83 | 46 | 3 | NR | 40 | 53 (6) | 46 (6) |
| 26 | 41 | 54 | CLL | FluMel-Campath | 83 | 12 | 3 | NR | NR | 51 (2) | 45 (2) |
| 2 | 82 | 56 | CLL/SLL | 2 Gy TBI ± Flu | 51 | 6 | 3 | 29* | 28 | 50 (5) | 39 (5) |
| 3 | 31 | 52 | DLBCL | 2 Gy TBI ± Flu | 72 | 44 | 4 | NR | 41 | 45 (3) | 35 (3) |
| 4 | 62 | 54 | Indolent (87% FL) | 2 Gy TBI ± Flu | 63 | 26 | 6 | 8 | 27 | 52 (3) | 43 (3) |
| 1 | 33 | 54 | MCL | 2 Gy TBI + Flu | 61 | 39 | 4 | 18* | 9† | 65 (2) | 60 (2) |
| 24 | 40 | 55 | Indolent (32), MCL (8) | Zevalin + Flu + 2 Gy TBI | 85 | 18 | 4 | 18 | NR | 51 (2) | 43 (2) |
| Current study | 40 | 58 | Indolent (18), DLBCL (14), MCL (8) | Zevalin + Flu + 2 Gy TBI | 15 | 0 | 6 | 43 | 63 | 54 (2) | 31 (2) |
| Reference . | n . | Age, y (median) . | Histology (no.) . | Regimen . | Chemosensitive, % . | CR at transplantation, % . | Prior treatment, no. . | Bulk > 5 cm, % . | HSCT-CI ≥ 3, % . | OS, % (y) . | PFS, % (y) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 47 | 53 | FL | FCR | 100 | 38 | 3 | NR | NR | 85 (5) | 83 (5) |
| 6 | 35 | 58 | MCL | PFA/FCR | 83 | 46 | 3 | NR | 40 | 53 (6) | 46 (6) |
| 26 | 41 | 54 | CLL | FluMel-Campath | 83 | 12 | 3 | NR | NR | 51 (2) | 45 (2) |
| 2 | 82 | 56 | CLL/SLL | 2 Gy TBI ± Flu | 51 | 6 | 3 | 29* | 28 | 50 (5) | 39 (5) |
| 3 | 31 | 52 | DLBCL | 2 Gy TBI ± Flu | 72 | 44 | 4 | NR | 41 | 45 (3) | 35 (3) |
| 4 | 62 | 54 | Indolent (87% FL) | 2 Gy TBI ± Flu | 63 | 26 | 6 | 8 | 27 | 52 (3) | 43 (3) |
| 1 | 33 | 54 | MCL | 2 Gy TBI + Flu | 61 | 39 | 4 | 18* | 9† | 65 (2) | 60 (2) |
| 24 | 40 | 55 | Indolent (32), MCL (8) | Zevalin + Flu + 2 Gy TBI | 85 | 18 | 4 | 18 | NR | 51 (2) | 43 (2) |
| Current study | 40 | 58 | Indolent (18), DLBCL (14), MCL (8) | Zevalin + Flu + 2 Gy TBI | 15 | 0 | 6 | 43 | 63 | 54 (2) | 31 (2) |
HSCT-CI indicates Hematopoietic Stem Cell Transplant Comorbidity Index22; PFA, cisplatin, fludarabine, and cytarabine; Flu, fludarabine; Mel, melphalan; and NR, not reported.
≥ 5 cm.
Charlson comorbidity scores.27
Even though we are encouraged by these results, not all patients achieved an early response; and for those that did not, the prospects of long-term disease-free survival were poor. For example, all 9 of the DLBCL patients who did not achieve at least a PR by 3 months after transplantation experienced disease progression, and only one survived longer than 18 months. One potential reason for the low response rate in these patients may be extensive prior use of R and the demonstrated potential for CD20 blocking, limiting our ability to optimally target tumor sites.28 This potential phenomenon is supported by the observation that the only CLL patient whose marrow was heavily infiltrated, yet not imageable, had immunophenotypic evidence of surface CD20 blocking. Future approaches might limit inclusion to those that have minimal measurable rituximab levels before therapy, or target an alternative antigen.28-30 Even in patients in whom CD20 targeting was optimized, the radiation dose to tumor sites could have been insufficient for disease control. Prior dosimetry data have suggested that the median absorbed dose to tumor with standard 90Y-ibritumomab tiuxetan is 1484 cGy, far lower than external beam radiotherapy doses required to sterilize disease sites.31,32 Improving tumor dose can be achieved by simple escalation of the RIT and by optimizing the tumor-to-normal organ ratios with methods, such as pretargeting.27,30,33
Until future refinements of NMAT can be made for such challenging clinical situations, these data represent a viable treatment option that can be currently offered to such patients. In summary, our study demonstrated the ability to safely induce early responses and prolonged survival in high-risk B-NHL patients with persistent disease that typically would not be considered candidates for NMAT. However, future work remains to optimize the RIT regimen and to conduct formal comparative studies to define the precise role of RIT in NMAT for lymphoma.
Presented in part at the 52nd Annual Meeting of the American Society of Hematology, Orlando, FL, December 5, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jennifer Roden, Tina Bogne, Jill Loveland, RN, Heather Nolting, RN, Greg Whitman, Lacey Hedin, the allogeneic transplant teams, and the patients and families who courageously participate in the advancement of knowledge.
This work was supported by the Leukemia & Lymphoma Society (SCOR grant 7040), Lymphoma Research Foundation Mantle Cell Lymphoma Research Initiative, Biogen-Idec, CLL Topics, the Mary A. Wright Memorial Research Fund, and a donation from Frank and Betty Vandermeer. A.K.G. is a Scholar in Clinical Research of the Leukemia & Lymphoma Society.
Authorship
Contribution: A.K.G., K.A.G., J.R., J.M.P., and O.W.P. conceived and designed the study; A.K.G. and O.W.P. provided administrative support; A.K.G., K.A.G., J.R., J.M.P., D.G.M., R.F.S., and O.W.P. provided study materials or patients; A.K.G., K.A.G., and G.O. collected and assembled data; A.K.G., K.A.G., G.O., J.R., M.C.M., and O.W.P. analyzed and interpreted data; A.K.G., K.A.G., and O.W.P. wrote the manuscript; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: A.K.G. has received research support from Biogen-Idec. D.G.M., O.W.P., and J.M.P. have served as paid consultants for Spectrum Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Ajay K. Gopal, University of Washington/Seattle Cancer Care Alliance, 825 Eastlake Ave E, G3-200, Seattle, WA 98195; e-mail: agopal@u.washington.edu.