Abstract
Recent studies have indicated a role of complement in regulating T-cell immunity but the mechanism of action of complement in this process remains to be clarified. Here we studied mice deficient in decay-accelerating factor (DAF), a key membrane complement regulator whose deficiency led to increased complement-dependent T-cell immune responses in vivo. By crossing OT-II and OT-I T-cell receptor transgenic mice with DAF-knockout mice, we found that lack of DAF on T cells did not affect their responses to antigen stimulation. Similarly, lack of DAF on antigen-presenting cells (APCs) of naive mice did not alter their T-cell stimulating activity. In contrast, APCs from DAF-knockout mice treated with inflammatory stimuli were found to be more potent T-cell stimulators than cells from similarly treated wild-type mice. Acquisition of higher T-cell stimulating activity by APCs in challenged DAF-knockout mice required C3 and C5aR and was correlated with decreased surface PD-L1 and/or increased CD40 expression. These findings implied that DAF suppressed T-cell immunity as a complement regulator in the context of inflammation but did not play an intrinsic role on T cells or APCs. Collectively, our data suggest a systemic and indirect role of complement in T-cell immunity.
Introduction
Decay-accelerating factor (DAF) is a glycosylphosphatidylinositol (GPI)–anchored cell surface regulator of complement activation. It inhibits complement activation by preventing the formation and accelerating the decay of C3/C5 convertases of both classic and alternative pathways.1 DAF is widely expressed on mammalian cells where its role in preventing complement injury is well established.1,2 In humans, deficiency of DAF on blood cells contributes to the pathogenesis of paroxysmal nocturnal hemoglobinuria, a syndrome characterized by uncontrolled complement-mediated hemolysis and platelet activation.3,4 In mice, gene deletion studies have shown that DAF-deficient (Daf−/−) animals were more susceptible to complement-mediated inflammatory injury in several disease models including anti-glomerular basement membrane glomerulonephritis, ischemia reperfusion injury and lupus dermatitis.5-8
In addition to its demonstrated activity in protecting host cells from complement attack, DAF has also been known to influence T-cell immune responses in vivo. For example, when WT and Daf−/− mice were immunized with ovalbumin in complete Freund's adjuvant, CD4+ T cells from Daf−/− mice were found to have an enhanced recall response to antigen restimulation and produced significantly higher levels of IFN-γ.9 Similar results in T-cell reactivity were obtained when mice were immunized with a pathogenic myelin oligodendrocyte glycoprotein (MOG) peptide which caused a more severe form of experimental autoimmune encephalomyelitis in Daf−/− mice.9 Likewise, in a model of CD8+ T-cell immune response to lymphocytic choriomeningitis virus infection, Daf−/− mice displayed a higher degree of CD8+ T-cell expansion in their spleens and lymph nodes, had more numbers of antigen-specific CD8+ T cells and cleared the virus more quickly.10 In other models involving skin and heart allograft transplantation experiments, deficiency of DAF from either the donor or the recipient mice elicited a more robust allogenic T-cell immune response, leading to accelerated rejection of the transplanted organs11,12
While the phenotype of enhanced T-cell immune response in Daf−/− mice has been demonstrated consistently, the mechanism by which DAF influences T-cell immunity in vivo remains to be fully elucidated. As a GPI-anchored protein in lipid raft of the plasma membrane, DAF may participate in the signaling pathways of the T-cell receptor (TCR).13 In addition, by serving as a cellular ligand for CD97, an EGF-TM7 receptor up-regulated rapidly on activated T cells, DAF could regulate T-cell activation and differentiation through complement-independent mechanisms.14,15 On the other hand, the fact that enhanced T-cell immune responses in Daf−/− mice required complement C3, and in many cases, C5a receptor (C5aR), supported a complement-dependent mechanism of action.9-12,16 It is not clear, however, whether DAF played an intrinsic role on T cells or antigen-presenting cells (APCs) during cognate T cell–APC interaction or indirectly affected APC and T-cell activation in vivo in the context of complement-mediated inflammation. In the present study, we have addressed this question by performing T–cell activation experiments using DAF-sufficient and -deficient TCR transgenic T cells and naive or activated APCs from WT and Daf−/− mice.
Methods
Mice and reagents
Daf−/−, Daf−/−/C3−/− and Daf−/−/C5aR−/− mice on C57BL/6 background were described previously.10,17 Wild-type (WT) C57BL/6 and BALB/c mice, OT-I and OT-II TCR transgenic mice were purchased from The Jackson Laboratory. OT-I and OT-II mice were crossed with Daf−/− mice to derive OT-I/ Daf−/− and OT-II/ Daf−/− mice, respectively. Sex- and age-matched WT and knockout or transgenic mice were used throughout this study. Mice were housed in a specific pathogen-free facility, and all experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Anti–mouse CD3 mAb (clone 145-2C11), anti–mouse CD28 mAb (clone 37.51) and ELISA kit for IFN-γ measurement were purchased from BD Pharmingen. Chicken ovalbumin (OVA) peptides (OVA257-264 and OVA323-339) were synthesized by Sigma-Aldrich. C5aR antagonist (AcF-[OPdChaWR]) was kindly provided by Dr John Lambris (University of Pennsylvania).18 Polyclonal rabbit anti–mouse DAF antibody was kindly provided by Dr Michael Holers (University of Colorado School of Medicine). Anti–mouse β-actin monoclonal antibody was from Sigma-Aldrich. The following antibodies used in cell staining were all from BD Pharmingen: Fc receptor blocking antibody (2.4G2), PE-rat anti–mouse CD11c mAb (HL3), PE-rat anti–mouse CD11b mAb (M1/70), FITC-rat anti–mouse IgG isotype control, FITC-rat anti–mouse CD40 (HM 40-3), FITC-rat anti–mouse CD80 (16-10A1), FITC-rat anti–mouse CD86 (B7-2), FITC-rat anti–mouse MHC-II (AF6-120.1), FITC-rat anti–mouse MHC-I (28-24-8), FITC-rat anti–mouse PD-L1 (MIH5) and FITC-hamster anti–mouse DAF (RIKO-5).
Cell purification
All reagents and columns used for cell isolation were purchased from Miltenyi Biotec. For purification of T cells and DCs, mouse spleens were minced and digested in 2 mg/mL of collagenase D (Roche Diagnostics) for 30 minutes at 37°C. Splenocytes were prepared by lyses of red blood cells with ACK buffer. CD4+ T cells, CD8+ T cells and CD11c+ dendritic cells were purified with CD4, CD8a or CD11c microbeads, respectively. The purity was approximately 95% for T cells and 90% for dendritic cells. Resident or elicited macrophages were collected from the peritoneal cavity by lavage with ice-cold PBS and CD11b+ macrophages were purified by CD11b microbeads. The purity was approximately 95%. Elicited macrophages were collected from mice 5 days after intraperitoneal injection of 2 mL of 3% thioglycollate medium (Becton Dickinson Microbiology System). In some experiments, splenic DCs were purified from mice 3 hours after intraperitoneal injection of lipopolysaccharide (LPS; 20 mg/kg; Sigma-Aldrich), alone or in combination with 30 μg/mouse of C5aR antagonist (LPS was injected after 10 minutes of C5aR antagonist administration). In other experiments, DCs isolated from naive WT and Daf−/− mice were stimulated with LPS (100 ng/mL) in vitro for 3 hours before mixing with purified T cells.
T-cell activation assays
For T-cell activation assays, unfractionated splenocytes, purified dendritic cells or macrophages were used as APCs as specified. Splenocytes were irradiated with 3000 Rad using a γ-irradiator. CD4+ T cells from OT-II mice or CD8+ T cells from OT-I mice were used as responders. In each well of a 96-well U-bottomed plate, 5 × 104 DCs or macrophages or 1 × 106 splenocytes were mixed with 2 × 105 T cells in 200μL complete medium (CM, DMEM containing 10% FBS, 2mM L-glutaime, 10mM Hepes, 0.1mM nonessential amino acids, 50μM 2-mercaptoethanol, 1mM sodium pyruvate and 100 U/mL penicillin-streptomycin). OVA peptides (OVA257-264 for OT-I CD8+ T cells and OVA323-339 for OT-II CD4+ T cells) were added at specified concentrations at the time of cell mixing. In alloreaction assay, 1 × 106 splenocytes from BALB/c mice were mixed with T cells from WT or Daf−/− mice (C57BL/6) at specified ratios of APC to T cells. Triplicate wells were set up for each condition. In some wells, 1μM C5aR antagonist was also added. After 60 hours of culture, 100 μL supernatant was collected for IFN-γ measurement by ELISA. For measuring T-cell proliferation, cultures were replenished with 100 μL fresh CM containing 1 μCi 3H-TdR and left for another 8 hours. Cultures were harvested by a cell harvester (Tomtec) and cell proliferation was measured by scintillation counting. In some experiments, purified T cells were activated by plate-bound anti–mouse CD3 mAb (5μg/mL) in the presence or absence of 2 μg/mL soluble anti–mouse CD28 mAb.
Analysis of cell surface markers
Expression of DAF, MHC-I, MHC-II, CD40, CD80, CD86, and PD-L1 on DCs and peritoneal macrophages were analyzed by flow cytometry. Cells (2 × 105) were first incubated with 0.5 μg Fc receptor blocking antibody (2.4G2) for 15 minutes at 4°C in 20 μL FACS buffer (PBS supplemented with 2% FCS, 0.1% sodium azide and 1mM EDTA); they were then stained with 0.25 μg PE-rat anti–mouse CD11c mAb (for DCs staining) or PE-rat anti–mouse CD11b mAb (for macrophages staining), in combination with one of the following FITC-conjugated antibodies (0.25 μg/staining): rat anti–mouse IgG isotype control, rat anti–mouse CD40, rat anti–mouse CD80, rat anti–mouse CD86, rat anti–mouse MHC-II, rat anti–mouse MHC-I, rat anti–mouse PD-L1 or hamster anti–mouse DAF in 50 μL FACS buffer for 30 minutes at 4°C. Cells were washed twice in staining buffer and fixed in 1% paraformaldehyde. Samples were analyzed on a FACSCalibur flow cytometer (BD Biosciences) and data were analyzed with the FlowJo software (Version 8.3; Tree Star).
Western blot analysis
Peritoneal macrophages were lysed in RIPA buffer and protein concentrations were determined by Bradford method. Total proteins (40 μg per well) were separated by SDS-PAGE under reducing conditions and then transferred to a polyvinylidene difluoride membrane (Millipore). The blot was probed with a polyclonal rabbit anti–mouse DAF antibody, and after stripping, was reprobed with a monoclonal anti–β-actin antibody. Signals were visualized by the ECL system (Amersham Bioscience) and detected by the FUJI image reader.
Statistical analysis
Data were expressed as mean ± SE. Groups were compared by Student t test and significance was defined as P < .05.
Results
DAF does not play an intrinsic role on T cells during T-cell activation
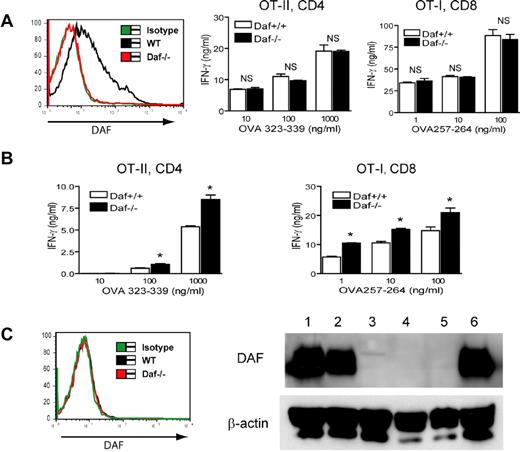
Previous in vivo studies using different models have clearly shown that DAF deficiency in mice promoted T-cell immunity and this phenotype was dependent on an intact complement system.9-12,16 To determine whether DAF plays an intrinsic role on T cells during antigen stimulation, we first used the OT-I and OT-II TCR transgenic mouse models and performed in vitro T-cell activation experiments. We crossed Daf−/− mice with OT-I and OT-II TCR transgenic mice and generated Daf−/−/OT-I and Daf−/−/OT-II mice. As shown in Figure 1A, DAF is highly expressed on transgenic CD8+ and CD4+ T cells of OT-I and OT-II mice, respectively, but is absent from transgenic T cells of Daf−/−/OT-I and Daf−/−/OT-II mice, as expected. CD8+ or CD4+ T cells were isolated from DAF-sufficient or DAF-deficient OT-I and OT-II mice and cocultured with irradiated WT mouse splenocytes as APCs in the presence of their cognate antigens, peptide OVA257-264 (for CD8+ T cells) or OVA323-339 (for CD4+ T cells), respectively. Figure 1B shows that WT and Daf−/− TCR transgenic T cells proliferated equally well in response to antigen stimulation. Furthermore, no difference in IFN-γ secretion was observed between the 2 types of T cells (Figure 1C). Similar results were obtained in experiments wherein the transgenic T cells were cocultured with irradiated splenocytes from Daf−/− mice as APCs (data not shown).
DAF expression on ovalbumin-specific TCR-transgenic T cells does not affect T-cell response to specific antigen stimulation. (A) FACS-staining analysis confirmed that both CD4+ and CD8+ T cells from Daf+/+ but not Daf−/− TCR transgenic mice expressed high levels of DAF on their surface. (B,C) Purified Daf+/+ or Daf−/− OT-II CD4+ (right panels) or OT-I CD8+ (left panels) T cells were cocultured with irradiated unfractionated splenocytes of C57BL/6 WT mice for 3 days in the presence of different concentrations of peptide OVA323-339 or OVA257-264, respectively. T-cell proliferation (B) was measured by 3H-TdR incorporation and IFN-γ production (C) was measured by ELISA. Data are representatives of 3 independent experiments. NS indicates not significant. P > .05.
DAF expression on ovalbumin-specific TCR-transgenic T cells does not affect T-cell response to specific antigen stimulation. (A) FACS-staining analysis confirmed that both CD4+ and CD8+ T cells from Daf+/+ but not Daf−/− TCR transgenic mice expressed high levels of DAF on their surface. (B,C) Purified Daf+/+ or Daf−/− OT-II CD4+ (right panels) or OT-I CD8+ (left panels) T cells were cocultured with irradiated unfractionated splenocytes of C57BL/6 WT mice for 3 days in the presence of different concentrations of peptide OVA323-339 or OVA257-264, respectively. T-cell proliferation (B) was measured by 3H-TdR incorporation and IFN-γ production (C) was measured by ELISA. Data are representatives of 3 independent experiments. NS indicates not significant. P > .05.
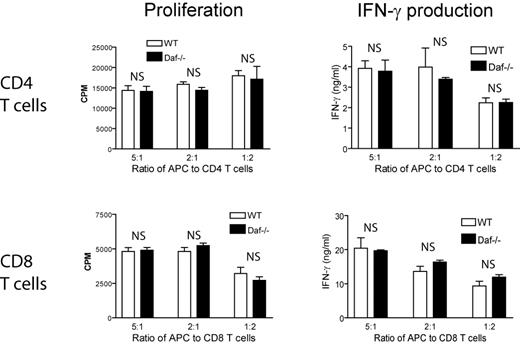
It is possible that an intrinsic effect of DAF on T cells was not easily revealed in the OT-I and OT-II TCR transgenic models where the T cells are clonal and express high affinity TCRs. We next performed allogeneic T-cell stimulation experiments using purified CD4+ or CD8+ T cells from C57BL/6 WT or Daf−/− mice and irradiated splenocytes from WT BALB/c mice. Figure 2 shows that responses of Daf−/−, CD4+, and CD8+ T cells to allogenic stimulation, as assessed by cell proliferation and IFN-γ production, were indistinguishable from that of WT T cells. In separate experiments, we also stimulated WT and Daf−/−, CD4+, and CD8+ T cells with plate-bound anti–mouse CD3 antibody in the presence or absence of anti–mouse CD28 antibody and again detected no difference in cell proliferation or IFN-γ production between WT and Daf−/− mouse T cells (data not shown). These results collectively suggested that DAF does not play an intrinsic role on mouse T cells during T-cell activation.
DAF expression on polyclonal T cells does not impact their response to allogenic antigen stimulation. Purified polyclonal CD4+ (top panels) or CD8+ (bottom panels) T cells from normal C57BL/6 WT or Daf−/− mice were cocultured at different ratios with irradiated splenocytes as APCs from BALB/c mice for 3 days. T-cell proliferation (left panels) was measured by 3H-TdR incorporation and IFN-γ production (right panels) was measured by ELISA. Data are representatives of 3 independent experiments. NS indicates not significant. P > .05.
DAF expression on polyclonal T cells does not impact their response to allogenic antigen stimulation. Purified polyclonal CD4+ (top panels) or CD8+ (bottom panels) T cells from normal C57BL/6 WT or Daf−/− mice were cocultured at different ratios with irradiated splenocytes as APCs from BALB/c mice for 3 days. T-cell proliferation (left panels) was measured by 3H-TdR incorporation and IFN-γ production (right panels) was measured by ELISA. Data are representatives of 3 independent experiments. NS indicates not significant. P > .05.
Thioglycollate-elicited but not resident peritoneal macrophages from Daf−/− mice are more potent T-cell stimulators
Given the previously observed T-cell immunity phenotype of Daf−/− mice9-12,16 and the finding that DAF does not play an intrinsic role on T cells, we hypothesized that DAF expression on APCs might affect T-cell activation and differentiation. To test this, we examined the activities of WT and Daf−/− mouse peritoneal macrophages as APCs. Figure 3A shows that DAF is expressed on resident peritoneal macrophages of WT but not Daf−/− mice. By in vitro T-cell stimulation assays, we detected no difference between the 2 types of resident macrophages in their ability to stimulate OT-II or OT-I TCR transgenic T cells (Figure 3A), suggesting that DAF also does not play an intrinsic role on APCs during cognate T cell–APC interaction. Interestingly, using the same assay system we obtained a different outcome with thioglycollate-elicited peritoneal macrophages, that is, cells from Daf−/− mice were more potent activators of TCR transgenic T cells than similarly elicited macrophages from WT mice (Figure 3B). Unexpectedly, we found that in contrast to resident peritoneal macrophages, elicited peritoneal macrophages from Daf−/− and WT mice were both negative for DAF expression, suggesting that thioglycollate challenge caused complete down-regulation of DAF expression on WT mouse macrophages (Figure 3C). Thus, while DAF deficiency enhanced the APC activity of elicited peritoneal macrophages, such a phenotype was not correlated with DAF expression on macrophages at the time of T cell-APC interaction.
Elicited but not resident peritoneal macrophages from Daf−/− mice are more potent T-cell stimulators than similarly harvested WT mouse macrophages. (A) FACS analysis showing that resident peritoneal macrophages from WT but not Daf−/− mice expressed DAF (left panel). No difference was observed between resident peritoneal macrophages of WT and Daf−/− mice in their ability to stimulate OT-II CD4+ or OT-I CD8+ T cells as measured by IFN-γ production. NS indicates not significant. P > .05. (B) Thioglycollate-elicited peritoneal macrophages from Daf−/− mice were more potent than similarly harvested WT macrophages in stimulating OT-II CD4+ or OT-I CD8+ T cells as measured by IFN-γ production. *P < .05. (C) FACS (left panel) and Western blot (right panel) analysis showing that DAF was completely down-regulated on thioglycollate-elicited WT mouse peritoneal macrophages. Lane designation in right panel: Lanes 1 and 6, WT splenocytes; lane 2, WT resident peritoneal macrophages; lane 3, Daf−/− resident peritoneal macrophages; lane 4, WT thioglycollate-elicited peritoneal macrophages; lane 5, Daf−/− thioglycollate-elicited peritoneal macrophages. Except Western blot data, all results are representatives of 5 independent experiments.
Elicited but not resident peritoneal macrophages from Daf−/− mice are more potent T-cell stimulators than similarly harvested WT mouse macrophages. (A) FACS analysis showing that resident peritoneal macrophages from WT but not Daf−/− mice expressed DAF (left panel). No difference was observed between resident peritoneal macrophages of WT and Daf−/− mice in their ability to stimulate OT-II CD4+ or OT-I CD8+ T cells as measured by IFN-γ production. NS indicates not significant. P > .05. (B) Thioglycollate-elicited peritoneal macrophages from Daf−/− mice were more potent than similarly harvested WT macrophages in stimulating OT-II CD4+ or OT-I CD8+ T cells as measured by IFN-γ production. *P < .05. (C) FACS (left panel) and Western blot (right panel) analysis showing that DAF was completely down-regulated on thioglycollate-elicited WT mouse peritoneal macrophages. Lane designation in right panel: Lanes 1 and 6, WT splenocytes; lane 2, WT resident peritoneal macrophages; lane 3, Daf−/− resident peritoneal macrophages; lane 4, WT thioglycollate-elicited peritoneal macrophages; lane 5, Daf−/− thioglycollate-elicited peritoneal macrophages. Except Western blot data, all results are representatives of 5 independent experiments.
Dendritic cells from LPS-treated but not naive Daf−/− mice were more potent T-cell stimulators
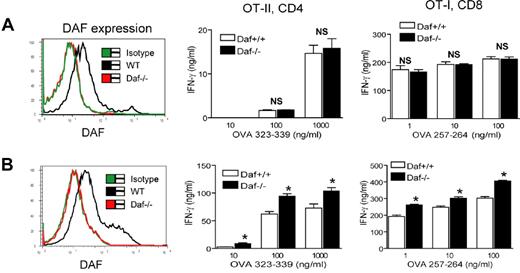
DCs are powerful professional APCs. We next investigated if DAF expression on DCs influences their T-cell stimulating activity. FACS-staining revealed that splenic DCs from naive WT mice expressed moderate level of DAF (Figure 4A). However, we found no difference between DCs from Daf−/− and WT mice in their activity to stimulate TCR-transgenic T cells as measured by IFN-γ production (Figure 4A). This result paralleled the data from the resident peritoneal macrophage experiment and further supported the conclusion that DAF does not play an intrinsic role on APCs during T-cell activation. To examine the function of DCs from Daf−/− mice challenged with inflammatory stimuli, we treated WT and Daf−/− mice with a sublethal dose of LPS. Figure 4B shows that LPS treatment did not appreciably affect DAF expression on WT splenic DCs (compare left panels in Figure 4A-B). Interestingly, we found that DCs isolated from LPS-treated Daf−/− mice were significantly more potent than DCs from similarly treated WT mice in stimulating T-cell activation (Figure 4B). This effect of DAF deficiency on DC function apparently required an in vivo environment as no difference was observed between WT and Daf−/− DCs stimulated by LPS in vitro (data not shown).
Splenic DCs from LPS-treated but not naive Daf−/− mice are more potent T-cell stimulators than similarly treated WT DCs. (A) FACS analysis (left panel) showing that splenic DCs from naive WT but not Daf−/− mice expressed DAF. There was no difference between splenic DCs from naive WT and Daf−/− mice in their ability to stimulate OT-II CD4+ or OT-I CD8+ T cells, as measured by IFN-γ production (right panel). NS indicates not significant. P > .05. (B) FACS analysis (left panel) showing that splenic DCs from LPS-treated WT but not Daf−/− mice expressed DAF. DCs from LPS-treated Daf−/− mice were more potent than similarly harvested WT mouse DCs in stimulating OT-II CD4+ or OT-I CD8+ T cells, as measured by IFN-γ production. *P < .05. All data are representative of 3 independent experiments.
Splenic DCs from LPS-treated but not naive Daf−/− mice are more potent T-cell stimulators than similarly treated WT DCs. (A) FACS analysis (left panel) showing that splenic DCs from naive WT but not Daf−/− mice expressed DAF. There was no difference between splenic DCs from naive WT and Daf−/− mice in their ability to stimulate OT-II CD4+ or OT-I CD8+ T cells, as measured by IFN-γ production (right panel). NS indicates not significant. P > .05. (B) FACS analysis (left panel) showing that splenic DCs from LPS-treated WT but not Daf−/− mice expressed DAF. DCs from LPS-treated Daf−/− mice were more potent than similarly harvested WT mouse DCs in stimulating OT-II CD4+ or OT-I CD8+ T cells, as measured by IFN-γ production. *P < .05. All data are representative of 3 independent experiments.
Acquisition of higher T-cell stimulating activities by Daf−/− macrophages and DCs in vivo required C3 and C5aR
We next performed experiments to dissect the mechanism by which thioglycollate-elicited peritoneal macrophages from Daf−/− mice and splenic DCs from LPS-treated Daf−/− mice became more potent APCs. We compared OT-II CD4+ T-cell responses stimulated by thioglycollate-elicited macrophages from WT, Daf−/−, Daf−/−/C3−/− or Daf−/−/C5aR−/− mice, respectively. Figure 5A shows that C3 or C5aR deletion in Daf−/− mice effectively abolished the phenotype of enhanced T-cell stimulating activity of their elicited macrophages, suggesting that in vivo complement activation, presumably caused by thioglycollate treatment, and C5aR-signaling was critical in producing the macrophage phenotype. In a similar experiment, we compared the potency of DCs from LPS-treated WT, Daf−/−, Daf−/−/C3−/− and Daf−/−/C5aR−/− mice to stimulate IFN-γ production by OT-II CD4+ T cells. As shown in Figure 5B, we found that acquisition of higher T-cell stimulating activity by DCs in LPS-treated Daf−/− mice also required C3 and C5aR. That C5aR signaling is critical for this process is further supported by the finding that pre-treatment of Daf−/− mice with a C5aR antagonist before LPS treatment likewise abolished the DC phenotype (Figure 5B). Importantly, addition of the C5aR antagonist in vitro (to the T cell/DC culture) had no effect (Figure 5B), suggesting that the effect of complement on DC function in LPS-treated mice is indirect and may have involved trans-cellular regulation.
Acquisition of higher T-cell stimulating activities by Daf−/− macrophages and DCs in vivo requires C3 and C5aR. (A) Thioglycollate-elicited peritoneal macrophages from Daf−/− mice, but not Daf−/−/C3−/− or Daf−/−/C5aR−/− mice, were more potent than similarly harvested WT macrophages in stimulating OT-II CD4+ T cells as measured by IFN-γ production. *P < .05 comparing with the Daf−/− group. (B) Splenic DCs from LPS-treated Daf−/− mice, but not similarly treated Daf−/−/C3−/− or Daf−/−/C5aR−/− mice or Daf−/− mice pre-treated with a C5aR antagonist (C5a-ant in vivo), were more potent than WT mouse DCs in stimulating OT-II CD4+ T cells as measured by IFN-γ production. *P < .05 comparing with the Daf−/− group. In contrast, addition of the C5aR antagonist to the cell culture of Daf−/− DCs and OT-II CD4+ T cells (C5a-ant in vitro) had no effect. NS indicates not significant. P > .05 comparing with the Daf−/− group.
Acquisition of higher T-cell stimulating activities by Daf−/− macrophages and DCs in vivo requires C3 and C5aR. (A) Thioglycollate-elicited peritoneal macrophages from Daf−/− mice, but not Daf−/−/C3−/− or Daf−/−/C5aR−/− mice, were more potent than similarly harvested WT macrophages in stimulating OT-II CD4+ T cells as measured by IFN-γ production. *P < .05 comparing with the Daf−/− group. (B) Splenic DCs from LPS-treated Daf−/− mice, but not similarly treated Daf−/−/C3−/− or Daf−/−/C5aR−/− mice or Daf−/− mice pre-treated with a C5aR antagonist (C5a-ant in vivo), were more potent than WT mouse DCs in stimulating OT-II CD4+ T cells as measured by IFN-γ production. *P < .05 comparing with the Daf−/− group. In contrast, addition of the C5aR antagonist to the cell culture of Daf−/− DCs and OT-II CD4+ T cells (C5a-ant in vitro) had no effect. NS indicates not significant. P > .05 comparing with the Daf−/− group.
Altered PD-L1 and CD40 expression on peritoneal macrophages and splenic DCs from thioglycollate- or LPS-treated Daf−/− mice
While resident peritoneal macrophages and naive splenic DCs from Daf−/− mice appeared to be normal, our data described in Figures 3 and 4 showed that macrophages and DCs from thioglycollate- or LPS-challenged Daf−/− mice functioned as more potent APCs. To understand why the latter cells have acquired such a phenotype, we performed FACS analysis of MHC and costimulatory molecule expression on the surface of DCs from LPS-treated WT, Daf−/−, Daf−/−/C3−/− and Daf−/−C5aR−/− mice. Although WT and Daf−/− mouse DCs expressed similar levels of MHC-I, MHC-II, CD80, CD86 and PD-L1 (data not shown), DCs from LPS-challenged Daf−/− mice expressed a significantly higher level of CD40 compared with WT DCs (Figure 6A). Importantly, this enhancement in CD40 expression was not observed on DCs from LPS-treated Daf−/−/C3−/− or Daf−/−C5aR−/− mice (Figure 6A).
Altered PD-L1 and CD40 expression on peritoneal macrophages and splenic DCs from thioglycollate- or LPS-treated Daf−/− mice. (A) FACS analysis of CD40 expression on splenic DCs from LPS-treated WT, Daf−/−, Daf−/−/C3−/− or Daf−/−/C5aR−/− mice. (B) FACS analysis of CD40 and PD-L1 expression on resident peritoneal macrophages from naive WT or Daf−/− mice. (C) FACS analysis of CD40 and PD-L1 expression on thioglycollate-elicited peritoneal macrophages from WT, Daf−/−, Daf−/−/C3−/− or Daf−/−/C5aR−/− mice. All data are representative of 3 independent experiments.
Altered PD-L1 and CD40 expression on peritoneal macrophages and splenic DCs from thioglycollate- or LPS-treated Daf−/− mice. (A) FACS analysis of CD40 expression on splenic DCs from LPS-treated WT, Daf−/−, Daf−/−/C3−/− or Daf−/−/C5aR−/− mice. (B) FACS analysis of CD40 and PD-L1 expression on resident peritoneal macrophages from naive WT or Daf−/− mice. (C) FACS analysis of CD40 and PD-L1 expression on thioglycollate-elicited peritoneal macrophages from WT, Daf−/−, Daf−/−/C3−/− or Daf−/−/C5aR−/− mice. All data are representative of 3 independent experiments.
We extended this analysis to resident and thioglycollate-elicited macrophages of WT and Daf−/− mice. No difference in expression of any of the MHC and costimulatory molecules on resident peritoneal macrophages was observed between WT and Daf−/− mice (Figure 6B and data not shown). Interestingly, we found that CD40 expression was significantly increased, whereas PD-L1 expression was significantly decreased, on thioglycollate-elicited peritoneal macrophages from Daf−/− mice compared with cells from WT, Daf−/−/C3−/− or Daf−/−/C5aR−/− mice (Figure 6C).
Discussion
There is accumulating evidence to support a role of complement in the regulation of T-cell immunity, and studies of Daf−/− mice have proven to be particularly instructive in this regard. DAF is a GPI-anchored complement regulator on the host cell surface. Previous studies in our laboratory and by others have shown that Daf−/− mice displayed enhanced T-cell immune responses in a variety of models including T-cell dependent autoimmunity, viral infection and allogeneic organ transplantation.9-12,16 These studies have also established that the T-cell immunity phenotype of Daf−/− mice was dependent on C3 and C5aR.9-12,16 Thus, although there is some literature suggesting that DAF may regulate T-cell immunity through complement-independent mechanisms, for example, as a GPI-anchored molecule in the lipid raft on T-cell membrane or as a ligand for the type II G protein-coupled cell surface receptor CD97, available data from Daf−/− mouse models suggested that DAF affected T-cell immune responses mainly through its regulatory activity on the complement system.
An important question then is whether DAF expression on T cells or APCs or both is important for its effect on T-cell activation. We addressed this question in the current study by using the OT-II and OT-I TCR transgenic mouse models. By crossing Daf−/− mice with OT-II or OT-I mice, we derived strains of mice in which the TCR transgenic CD4+ or CD8+ T cells were deficient in DAF. Using these mice and by in vitro T-cell activation assays with OVA peptides as antigens, we found that DAF does not play an intrinsic role on T cells during specific antigen-induced T-cell activation. We further established that DAF expression on polyclonal T cells also did not play a detectible role when such T cells were activated by allogenic antigens or by anti-CD3/anti-CD28 mAbs.
In separate experiments, we also revealed that DAF expression on resident peritoneal macrophages or splenic DCs from naive mice had no effect on their activity to stimulate OT-II or OT-I T cells. Notwithstanding this, we found that thioglycollate-elicited peritoneal macrophages from Daf−/− mice and splenic DCs from LPS-challenged Daf−/− mice were more potent APCs than similarly harvested cells from WT mice. The result concerning elicited peritoneal macrophages was consistent with data described by others who observed a similar enhanced T-cell stimulating function of such cells from Daf−/− mice.19 This finding was taken as supporting evidence for the theory that local complement activation within the immunologic synapse occurs during cognate T cell–APC engagement and serves as a costimulatory signal to enhance T-cell activation.16,20,21 It was postulated that in the absence of DAF, there is a higher degree of complement activation within the immunologic synapse, leading to increased anaphylatoxin production and enhanced T-cell activation.16 Based on the data presented here, we offer an alternative interpretation of the phenotype seen with the elicited peritoneal macrophages of Daf−/− mice. We showed that DAF is completely down regulated on elicited macrophages of WT mice. Thus, at the time of T-cell engagement with elicited macrophages in the in vitro T-cell activation assays, there was no difference between WT and Daf−/− macrophages in their surface DAF levels because both types of cells were devoid of DAF expression. Accordingly, the enhanced T-cell stimulating function of Daf−/− macrophages could not be attributed to increased local complement activation within the immunologic synapse. Rather, our data suggested that elicited peritoneal macrophages from Daf−/− mice were already phenotypically different from the WT macrophages in that they had higher CD40 expression and lower PD-L1 expression. CD40 and PD-L1 help to deliver positive and negative costimualtory signals, respectively, during T cell–APC interaction and the observed changes in their expression levels most likely contributed to the higher T-cell stimulating activity of the elicited macrophages of Daf−/− mice. Likewise, increased CD40 expression, rather than deficiency of DAF, is presumably responsible for the higher T-cell stimulating activity of splenic DCs from LPS-treated Daf−/− mice. DCs from naive Daf−/− mice had normal CD40 expression (data not shown) and were indistinguishable from WT DCs in their potency to stimulate T cells even though they lacked DAF expression.
How thioglycollate or LPS challenge caused decreased PD-L1 and/or increased CD40 expression on peritoneal macrophages and splenic DCs of Daf−/− mice remains to be established. The fact that both C3 and C5aR are required implied that thioglycollate and LPS22 exerted their effects in vivo by activating complement to release the anaphylatoxin C5a which then acted as an inflammatory mediator. Because splenic DCs in mice do not express C5aR,23 C5aR-dependent acquisition of a higher T-cell stimulating activity by DCs in LPS-treated Daf−/− mice must have involved inter-cellular communication between DCs and other C5aR-expressiing cell types. Another issue waiting to be clarified is the mechanism by which thioglycollate dramatically down regulated DAF expression on peritoneal macrophages. Regardless, our present study has suggested that DAF suppresses T-cell immunity in the context of systemic complement activation and inflammation but does not play an intrinsic role either on T cells or APCs during T cell–APC interaction. This conclusion may bear relevance to future effort in targeting the complement system for the treatment of T-cell mediated human immune disorders.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr John Lambris for C5aR antagonist, Dr Michael Holers for rabbit anti–mouse DAF antibodies and Dr Craig Gerard for C5aR−/− mice.
This work was supported by National Institutes of Health grants AI44970, AI49344 and AI63288.
National Institutes of Health
Authorship
Contribution: C.F., T.M. and W.-C. S. designed experiments and analyzed data; C.F. and T.M performed experiments; and C.F. and W.-C. S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Wen-Chao Song, Institute for Translational Medicine and Therapeutics and Department of Pharmacology, University of Pennsylvania School of Medicine, Rm 1254 BRBII/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: songwe@upenn.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal