Abstract
The link between EBV infection and Burkitt lymphoma (BL) is strong, but the mechanism underlying that link has been elusive. We have developed a mouse model for EBV-associated BL in which LMP2A, an EBV latency protein, and MYC are expressed in B cells. Our model has demonstrated the ability of LMP2A to accelerate tumor onset, increase spleen size, and bypass p53 inactivation. Here we describe the results of total gene expression analysis of tumor and pretumor B cells from our transgenic mouse model. Although we see many phenotypic differences and changes in gene expression in pretumor B cells, the transcriptional profiles of tumor cells from LMP2A/λ-MYC and λ-MYC mice are strikingly similar, with fewer than 20 genes differentially expressed. We evaluated the functional significance of one of the most interesting differentially expressed genes, Egr1, and found that it was not required for acceleration of tumor onset by LMP2A. Our studies demonstrate the remarkable ability of LMP2A to affect the pretumor B-cell phenotype and tumorigenesis without substantially altering gene expression in tumor cells.
Introduction
The connection between Epstein-Barr virus (EBV) and Burkitt lymphoma (BL) led to identification of the virus 50 years ago, but the mechanistic links between EBV and BL are still unclear. Epidemiologic data reveal a strong association between EBV and BL in regions of equatorial Africa where BL is endemic and has a high incidence rate. EBV genomes can be found in nearly 100% of endemic BL tumors. BL is also found at a lower incidence worldwide in a form known as sporadic BL. Sporadic BL has a lower association with EBV infection, with 15% to 85% of tumors containing the virus, varying by region.1 BL is also a disease seen in patients infected with HIV, where it has an intermediate association with EBV infection. Regardless of the presence of EBV, all forms of BL contain a translocation between the proto-oncogene c-MYC and an Ig locus2,3 resulting in deregulated expression of MYC in B lymphocytes. Clinically, a BL diagnosis is based on the presence of the MYC translocation, a “starry sky” histomorphology, which is the result of high levels of apoptosis caused by overexpression of MYC in tumor cells, and immunophenotypic markers, such as CD10 and Bcl6. Distinguishing between BL and diffuse large B cell lymphoma (DLBCL) can be difficult because the diagnostic characteristics of the two tumors are quite similar. Identification of gene expression signatures has proven to be a very effective way to distinguish between the two lymphoma types.4,5
Extensive studies comparing EBV+ and EBV− BL tumors are lacking. Until recently, the only existing data were based on BL cell lines propagated in culture where they can acquire additional molecular changes and may not be an accurate representation of the molecular events that lead to tumorigenesis in vivo. However, a recent study compared gene expression profiles among BL subtypes, including endemic, sporadic, and HIV-associated BL samples. Although the BL samples were clearly distinct from other B-cell malignancies, distinguishing among BL subtypes was less precise, and the samples did not cluster according to EBV status.6 Therefore, the contributions of EBV to BL development are still not clear.
The difficulty in elucidating the role of EBV in BL pathogenesis can partly be attributed to limited viral gene expression in BL tumors. EBV is known to adopt different patterns of latent viral gene expression in infected cell lines and EBV-positive tumors. The only viral protein that is easily detectable in BL tumor cells is EBNA1, whose main function is to maintain the viral genome in an infected cell.7 However, careful analyses have detected low levels of another EBV latency protein, latent membrane protein 2A (LMP2A) in BL biopsies.8,9 LMP2A is a membrane protein containing an amino terminal cytoplasmic tail that provides signaling capability. Tyrosine residues in the amino terminus have been shown to interact with some of the same signaling molecules that bind the B-cell receptor (BCR), such as the protein tyrosine kinases Lyn and Syk.10,11 Many lines of evidence demonstrate the pro-survival function of LMP2A, including the ability of LMP2A to activate the Ras/PI3K/Akt pathway,12-14 the MAP kinase pathway,15 NF-κB,16 and Bcl-2 and Bcl-XL.13
Transgenic mice in which MYC is expressed from an Ig promoter have been used as a model to study BL pathogenesis.17-19 These mice develop a fatal lymphoma within several months. We developed a transgenic mouse model to study the role of LMP2A in BL20,21 by crossing λ-MYC transgenic mice18 with mice that express LMP2A in B cells.22,23 LMP2A/λ-MYC mice also develop tumors, and tumor onset is accelerated compared with λ-MYC mice in two LMP2A transgenic lines.20,21 Alteration of the major tumor suppressor p53 is common in tumors from MYC transgenic mice.19 However, the p53 pathway was functionally intact in LMP2A/λ-MYC tumor cells.20 To investigate the functions of LMP2A in the context of deregulated MYC and understand mechanism of acceleration of tumor onset and p53 bypass by LMP2A in our mouse model of BL, we analyzed total gene expression in both tumor and pretumor B cells from λ-MYC and LMP2A/λ-MYC mice. In comparing expression profiles between pretumor B cells from λ-MYC and LMP2A/λ-MYC mice, a large number of genes were significantly differentially expressed. Remarkably, comparison of λ-MYC and LMP2A/λ-MYC tumor B cells revealed very limited differences in gene expression. Furthermore, one of the most significantly differentially expressed genes, the transcription factor Egr1, does not appear to be required for the phenotype of the LMP2A/λ-MYC mice. These data support a model in which LMP2A influences MYC-induced lymphomagenesis before progression to malignancy, and may do so post-translationally.
Methods
Mice
Construction and characterization of the EμLMP2A transgenic mice have been described previously.22,23 The EμLMP2A Tg6 line23 was used in all experiments. Double transgenic mice were generated by crossing the Tg6-LMP2A line with previously characterized λ-MYC mice18 and dominant negative Egr1 (DN Egr) transgenic mice.24 Mice were sacrificed when cervical lymph node tumors could be observed externally and mice were moribund. Animals were maintained at Northwestern University's Center for Comparative Medicine, in accordance with University animal welfare guidelines, and approval was granted by the Northwestern University Animal Care and Use Committee.
Tumor and spleen cell isolation
Pretumor spleens were isolated from 3-week-old mice. Magnetic activated cell sorting using CD19+ selection and negative selection B-cell isolation kits were used to purify B cells (Miltenyi Biotec). Pretumor spleens (those used for FACS analysis of total splenocytes) and cervical lymph node tumors were dissociated between frosted glass slides and treated with 155mM ammonium chloride to lyse red blood cells.
Flow cytometry
Total pretumor splenocytes and cervical lymph node tumor cells were stained with B220-allophycocyanin (APC), IgM-PE, or CD43-PE fluorochrome-conjugated antibodies (BD Biosciences). Purified B cells from pretumor spleens were stained with B220-APC, fixed with 1% paraformaldehyde, and stored in 70% ethanol at −20°C overnight. Cells were then stained with propidium iodide/RNase staining buffer according to the manufacturer's instructions (BD Biosciences). All samples were analyzed using the LSRII flow cytometer and FACS Diva Version 6.1 software (BD Biosciences).
Histology
Spleens from 3-week-old mice or cervical lymph node tumors were fixed in 10% buffered formalin phosphate (Fisher Scientific) followed by paraffin embedding. Tissues were sectioned and stained with hematoxylin and eosin at the Mouse Histology and Phenotyping Laboratory at Northwestern University. Stained tissue sections were imaged using a Leica DMR upright microscope and AxioCam MRC camera with AxioVision Rel 4.6 acquisition software.
RNA preparation and microarray experiments
Total RNA was isolated from tumor or pretumor cells using RNeasy RNA extraction kit from QIAGEN. RNA samples were amplified, labeled, and hybridized at the Genomics Core Facility at the Center for Genetic Medicine at Northwestern University according to Illumina protocols. MouseRef-8 Version 2.0 Expression Bead Chips (Illumina) were used for all experiments. An Illumina iScan was used to scan the arrays. The primary expression profiling data are available at the National Center for Biotechnology Information Gene Expression Omnibus under the accession number GSE26918.
Statistical analysis
GeneSpringGX analysis software (Agilent Technologies) was used for the microarray data analysis. The quantile normalization method was used for preprocessing, and the baseline was set to the median of all samples. The detection P value was used to eliminate probes that were not significantly expressed in any sample. Probes were excluded from the analysis if none of the samples had a detection P value > .95. To identify significant probes, the Benjamini-Hochberg false discovery rate was used for the multiple testing correction P value. Probes with a P value < .05 and a fold change of > 1.5 were considered significant.
Real time RT-PCR
Total RNA was isolated from tumor or pretumor cells using RNeasy RNA extraction kit from QIAGEN. Reverse transcription using a High-Capacity cDNA Reverse Transcription kit from Applied Biosystems was used to generate cDNA. Real-time PCR was carried out on Applied Biosystems Step One Plus machine using primers specific for the indicated gene as well as Hprt or Gapdh. Fast SYBR Green master mix from Applied Biosystems was used in all reactions. The ΔΔCt method was used to normalize gene expression to Hprt or Gapdh.
Immunoblots
Tumor cells were lysed in modified RIPA buffer (0.1M Tris-HCl pH 8.2, 0.15M NaCl, 2% SDS, 1% NP40 alternate, 0.5% Na-deoxycholate, 0.01M NaF, 0.002M Na3VO4, 0.002M phenylmethylsulfonyl floride, 0.01M DTT) with protease and phosphatase inhibitor cocktails (Roche Diagnostics). DNA and nucleic acid were digested with Benzonase nuclease (Sigma-Aldrich). Lysates were cleared and heated for 10 minutes at 72°C and then electrophoretically separated by 10% SDS-PAGE. Protein was transferred to Immobilon-P membrane (Millipore) and probed for Egr1 and Gapdh (Abcam).
Results
The EBV-associated BL mouse model phenotype
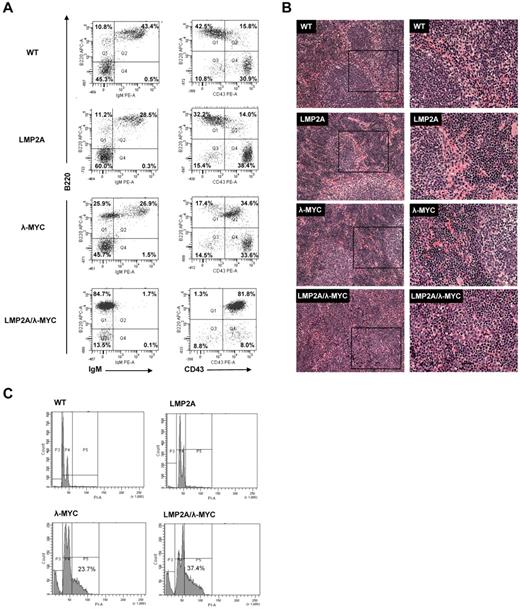
To study the contributions of EBV LMP2A to BL pathogenesis, we have used two LMP2A transgenic mouse models. In the TgE-LMP2A transgenic line, LMP2A is expressed at a high level.23 The TgE line demonstrates altered B-cell development, as expression of LMP2A allows survival of IgM− cells in the periphery.22 The Tg6-LMP2A transgenic line expresses lower levels of LMP2A, and B-cell development is normal in these mice.23,25 Using either of these models, LMP2A accelerates tumor onset in λ-MYC transgenic mice.20,21 In this report, we focus on the Tg6-LMP2A transgenic line, from here simply referred to as LMP2A transgenic. Although B-cell development is normal in Tg6-LMP2A transgenic mice23 (Figure 1A), crossing the LMP2A transgenic line with λ-MYC transgenic mice results in an altered developmental phenotype. Figure 1A shows the cell surface phenotype of splenocytes from 3-week-old littermates. In wild-type and LMP2A transgenic mice, most B220+ splenocytes also express IgM. However, B220+ B cells do not express high levels of CD43, a marker expressed on pro-B and pre-BI but down-regulated on pre-BII cells (Figure 1A). At 3 weeks of age, development is slightly delayed in the λ-MYC mice, where the spleens contain relatively fewer IgM+ B cells and more CD43+ B cells (Figure 1A). By 6 weeks of age, the immunophenotype of λ-MYC B cells is not different from wild-type (data not shown). We have previously reported that LMP2A/λ-MYC mice have enlarged spleens,20,21 which can be observed as early as 3 weeks of age. In contrast to wild-type, LMP2A transgenic, or λ-MYC mice, B220+ B cells in LMP2A/λ-MYC spleens lack expression of IgM and show high CD43 expression (Figure 1A). Figure 1A shows representative plots from individual mice. Tabulated data on the average percentage of cells that fall into B220+, IgM−, or CD43− (quadrant 1), B220+, IgM+, or CD43+ (quadrant 2), B220−, IgM−, or CD43− (quadrant 3), and B220−, IgM+, or CD43+ (quadrant 4) for 7 to 14 mice per genotype is shown in Tables 1 and 2.
Pretumor transgenic mouse phenotypes. (A) Total splenocytes were harvested from 3-week-old littermates, stained with B220-APC (y-axis) and IgM-PE or CD43-PE (x-axis), and analyzed by flow cytometry. The percentage B220+, IgM−, or CD43− (Q1), B220+, IgM+, or CD43+ (Q2), B220−, IgM−, or CD43− (Q3), and B220−, IgM+, or CD43+ (Q4) is indicated for each genotype. Data are presented for individual mice but are representative of 7 to 14 mice analyzed per genotype (see also summarized data in Tables 1 and 2). (B) Spleens of the indicated genotype were fixed in 10% buffered formalin phosphate followed by paraffin embedding. Spleens were then sectioned and stained with H&E. The beginning of normal splenic structures can be observed (see arrows), with the LMP2A/λ-MYC spleen less organized. The rectangles in the left-hand column represent the area that is magnified in the right-hand column. (C) Spleens were isolated from 3-week-old littermates, and B cells were purified using a MACS protocol with magnetic beads specific for the B-cell marker CD19. Purified B cells were fixed with paraformaldehyde and ethanol and then stained with propidium iodide (x-axis) to measure the percentage of cycling cells. Cells were analyzed by flow cytometry to measure the percentage of cells that fall into the sub G0, G0/G1, or S/G2/M gates.
Pretumor transgenic mouse phenotypes. (A) Total splenocytes were harvested from 3-week-old littermates, stained with B220-APC (y-axis) and IgM-PE or CD43-PE (x-axis), and analyzed by flow cytometry. The percentage B220+, IgM−, or CD43− (Q1), B220+, IgM+, or CD43+ (Q2), B220−, IgM−, or CD43− (Q3), and B220−, IgM+, or CD43+ (Q4) is indicated for each genotype. Data are presented for individual mice but are representative of 7 to 14 mice analyzed per genotype (see also summarized data in Tables 1 and 2). (B) Spleens of the indicated genotype were fixed in 10% buffered formalin phosphate followed by paraffin embedding. Spleens were then sectioned and stained with H&E. The beginning of normal splenic structures can be observed (see arrows), with the LMP2A/λ-MYC spleen less organized. The rectangles in the left-hand column represent the area that is magnified in the right-hand column. (C) Spleens were isolated from 3-week-old littermates, and B cells were purified using a MACS protocol with magnetic beads specific for the B-cell marker CD19. Purified B cells were fixed with paraformaldehyde and ethanol and then stained with propidium iodide (x-axis) to measure the percentage of cycling cells. Cells were analyzed by flow cytometry to measure the percentage of cells that fall into the sub G0, G0/G1, or S/G2/M gates.
Flow cytometric analysis of total splenocytes from 3-week-old mice: IgM and B220
| . | Q1: B220+, IgM− . | Q2: B220+, IgM+ . | Q3: B220−, IgM− . | Q4: B220−, IgM+ . |
|---|---|---|---|---|
| WT (n = 7) | 14.8 ± 6.4 | 42.6 ± 7.4 | 41.7 ± 9.0 | 0.9 ± 0.5 |
| Tg6 LMP2A (n = 13) | 12.3 ± 4.3 | 30.6 ± 11.0 | 56.2 ± 10.4 | 0.8 ± 0.4 |
| λ-MYC (n = 14) | 30.5 ± 10.5 | 22.8 ± 5.9 | 45.7 ± 13.4 | 1.0 ± 0.9 |
| LMP2A/λ-MYC (n = 14) | 73.5 ± 10.9 | 1.2 ± 1.0 | 25.0 ± 11.2 | 0.3 ± 0.2 |
| . | Q1: B220+, IgM− . | Q2: B220+, IgM+ . | Q3: B220−, IgM− . | Q4: B220−, IgM+ . |
|---|---|---|---|---|
| WT (n = 7) | 14.8 ± 6.4 | 42.6 ± 7.4 | 41.7 ± 9.0 | 0.9 ± 0.5 |
| Tg6 LMP2A (n = 13) | 12.3 ± 4.3 | 30.6 ± 11.0 | 56.2 ± 10.4 | 0.8 ± 0.4 |
| λ-MYC (n = 14) | 30.5 ± 10.5 | 22.8 ± 5.9 | 45.7 ± 13.4 | 1.0 ± 0.9 |
| LMP2A/λ-MYC (n = 14) | 73.5 ± 10.9 | 1.2 ± 1.0 | 25.0 ± 11.2 | 0.3 ± 0.2 |
Values are percentages.
Flow cytometric analysis of total splenocytes from 3-week-old mice: CD43 and B220
| . | Q1: B220+, CD43− . | Q2: B220+,CD43+ . | Q3: B220−, CD43− . | Q4: B220−, CD43+ . |
|---|---|---|---|---|
| WT (n = 6) | 47.0 ± 4.7 | 13.6 ± 5.0 | 15.1 ± 9.9 | 24.3 ± 6.8 |
| Tg6 LMP2A (n = 12) | 33.7 ± 7.4 | 10.0 ± 3.3 | 19.2 ± 12.3 | 37.2 ± 10.0 |
| λ-MYC (n = 10) | 24.5 ± 9.8 | 30.2 ± 9.8 | 20.0 ± 8.7 | 25.3 ± 11.9 |
| LMP2A/λ-MYC (n = 12) | 3.4 ± 1.8 | 70.9 ± 11.4 | 14.6 ± 6.3 | 11.2 ± 6.3 |
| . | Q1: B220+, CD43− . | Q2: B220+,CD43+ . | Q3: B220−, CD43− . | Q4: B220−, CD43+ . |
|---|---|---|---|---|
| WT (n = 6) | 47.0 ± 4.7 | 13.6 ± 5.0 | 15.1 ± 9.9 | 24.3 ± 6.8 |
| Tg6 LMP2A (n = 12) | 33.7 ± 7.4 | 10.0 ± 3.3 | 19.2 ± 12.3 | 37.2 ± 10.0 |
| λ-MYC (n = 10) | 24.5 ± 9.8 | 30.2 ± 9.8 | 20.0 ± 8.7 | 25.3 ± 11.9 |
| LMP2A/λ-MYC (n = 12) | 3.4 ± 1.8 | 70.9 ± 11.4 | 14.6 ± 6.3 | 11.2 ± 6.3 |
Values are percentages.
Splenic architecture is altered in LMP2A/λ-MYC mice. H&E staining of spleen sections from 3-week-old littermates reveal organized structures, and the beginnings of follicle development can be observed in wild-type, LMP2A transgenic, and λ-MYC mice (Figure 1B). LMP2A/λ-MYC spleens, however, are much more disorganized, and follicle structures are rarely observed (Figure 1B). Examination of cell cycle profiles in purified B cells from transgenic littermates also revealed differences in LMP2A/λ-MYC mice. B cells from both LMP2A/λ-MYC and λ-MYC mice show a considerable population of cycling cells with DNA content greater than 2n consistent with the MYC transgene driving cell cycle progression. However, a greater proportion of B cells from LMP2A/λ-MYC spleens fall into the S/G2/M gate of proliferating cells (Figure 1C). These data are consistent with a previous report of TgE-LMP2A/λ-MYC mice.21
The EBV-associated BL tumor phenotype
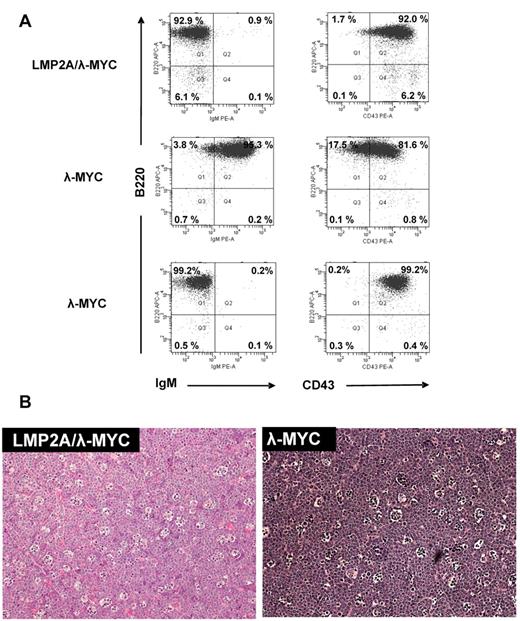
Cervical lymph node tumor cells from LMP2A/λ-MYC mice are nearly all B220+ B lymphocytes (Figure 2A). The immunophenotype of the tumor cells is similar to B cells from the spleens of 3-week-old mice in that the tumor B cells express no surface IgM and high levels of CD43 (Figure 2A). Tumors from λ-MYC mice are also nearly all B220+ B cells. The λ-MYC tumor cells are often IgM+, consistent with previous reports,18 although we also observe IgM− λ-MYC tumors (Figure 2A). Tables 3 and 4 show tabulated data on the average percentage of cells that fall into B220+, IgM− or CD43− (quadrant 1), B220+, IgM+ or CD43+ (quadrant 2), B220−, IgM−, or CD43− (quadrant 3), and B220−, IgM+, or CD43+ (quadrant 4) for cervical lymph node tumors. More than 90% of tumor cells from LMP2A/λ-MYC mice (n = 18) are B220+, IgM− (Tables 3 and 4).
Phenotype of transgenic mouse tumors. (A) Cervical lymph node tumor cells were harvested from tumor-burdened mice and stained with B220-APC (y-axis) and IgM-PE or CD43-PE (x-axis) and analyzed by flow cytometry. The percentage of cells that fall within each quadrant is indicated. Both IgM+ (n = 7) and IgM− (n = 5) λ-MYC tumors are observed, and an example of each is shown. All LMP2A/λ-MYC tumors are IgM− (n = 18; see also summarized quadrant data in Tables 3 and 4). (B) Cervical lymph node tumors were isolated from tumor-burdened mice and fixed in 10% buffered formalin phosphate followed by paraffin embedding. Tumors were then sectioned and stained with hematoxylin and eosin. Note the “starry sky” pattern that is also characteristic of human Burkitt lymphoma tumors.
Phenotype of transgenic mouse tumors. (A) Cervical lymph node tumor cells were harvested from tumor-burdened mice and stained with B220-APC (y-axis) and IgM-PE or CD43-PE (x-axis) and analyzed by flow cytometry. The percentage of cells that fall within each quadrant is indicated. Both IgM+ (n = 7) and IgM− (n = 5) λ-MYC tumors are observed, and an example of each is shown. All LMP2A/λ-MYC tumors are IgM− (n = 18; see also summarized quadrant data in Tables 3 and 4). (B) Cervical lymph node tumors were isolated from tumor-burdened mice and fixed in 10% buffered formalin phosphate followed by paraffin embedding. Tumors were then sectioned and stained with hematoxylin and eosin. Note the “starry sky” pattern that is also characteristic of human Burkitt lymphoma tumors.
Flow cytometric analysis of cervical lymph node tumor cells: IgM and B220
| . | Q1: B220+, IgM− . | Q2: B220+, IgM+ . | Q3: B220−, IgM− . | Q4: B220−, IgM+ . |
|---|---|---|---|---|
| LMP2A/λ-MYC CT (n = 18) | 95.4 ± 3.4 | 0.8 ± 0.6 | 3.7 ± 3.3 | 0.1 ± 0.1 |
| λ-MYC CT (IgM+) (n = 7) | 30.7 ± 26.4 | 68.2 ± 25.7 | 1.0 ± 0.9 | 0.2 ± 0.2 |
| λ-MYC CT (IgM−) (n = 5) | 96.7 ± 2.6 | 1.3 ± 1.5 | 1.8 ± 1.8 | 0.1 ± 0.1 |
| . | Q1: B220+, IgM− . | Q2: B220+, IgM+ . | Q3: B220−, IgM− . | Q4: B220−, IgM+ . |
|---|---|---|---|---|
| LMP2A/λ-MYC CT (n = 18) | 95.4 ± 3.4 | 0.8 ± 0.6 | 3.7 ± 3.3 | 0.1 ± 0.1 |
| λ-MYC CT (IgM+) (n = 7) | 30.7 ± 26.4 | 68.2 ± 25.7 | 1.0 ± 0.9 | 0.2 ± 0.2 |
| λ-MYC CT (IgM−) (n = 5) | 96.7 ± 2.6 | 1.3 ± 1.5 | 1.8 ± 1.8 | 0.1 ± 0.1 |
Values are percentages.
Flow cytometric analysis of cervical lymph node tumor cells: CD43 and B220
| . | Q1: B220+, CD43− . | Q2: B220+, CD43+ . | Q3: B220−, CD43− . | Q4: B220−, CD43+ . |
|---|---|---|---|---|
| LMP2A/λ-MYC CT (n = 17) | 9.4 ± 10.2 | 86.8 ± 10.0 | 0.3 ± 0.3 | 3.5 ± 3.7 |
| λ-MYC CT (IgM+) (n = 7) | 14.4 ± 27.3 | 83.9 ± 27.4 | 0.2 ± 0.4 | 1.5 ± 1.8 |
| λ-MYC CT (IgM−) (n = 5) | 3.8 ± 3.6 | 93.6 ± 4.1 | 0.4 ± 0.4 | 2.1 ± 1.9 |
| . | Q1: B220+, CD43− . | Q2: B220+, CD43+ . | Q3: B220−, CD43− . | Q4: B220−, CD43+ . |
|---|---|---|---|---|
| LMP2A/λ-MYC CT (n = 17) | 9.4 ± 10.2 | 86.8 ± 10.0 | 0.3 ± 0.3 | 3.5 ± 3.7 |
| λ-MYC CT (IgM+) (n = 7) | 14.4 ± 27.3 | 83.9 ± 27.4 | 0.2 ± 0.4 | 1.5 ± 1.8 |
| λ-MYC CT (IgM−) (n = 5) | 3.8 ± 3.6 | 93.6 ± 4.1 | 0.4 ± 0.4 | 2.1 ± 1.9 |
Values are percentages.
The histology of the LMP2A/λ-MYC and λ-MYC tumors is of particular interest. H&E-stained tissue sections of tumors from both LMP2A/λ-MYC and λ-MYC genotypes display a “starry sky” pattern that is similar to the histopathology of human BL tumors (Figure 2B).18 The “starry sky” appearance is not seen in spleens from 3-week-old littermates (Figure 1B). Importantly, we can use the same criteria used by clinicians to diagnose BL in human patients to distinguish between “tumor” and “pretumor” animals in our mouse model. In our analysis, “pretumor” B cells are isolated from the spleens of 3-week-old mice in which the “starry sky” histopathology is absent. We define “tumor” B cells as those isolated from the cervical lymph node of tumor-burdened transgenic mice in which the “starry sky” phenotype is observed.
Expression profiling of EBV-associated and non–EBV-associated BL
Illumina gene expression arrays were used to analyze total gene expression in B cells from our transgenic mice. RNA from both tumor and pretumor samples were included in the analysis. B cells were purified from the spleens of 3-week-old (pretumor) mice, and tumor B cells were isolated from the cervical lymph nodes of tumor-bearing mice. Both IgM+ and IgM− λ-MYC tumor samples were included. The gene array chips purchased from Illumina contained approximately 25 000 probe sequences corresponding to more than 19 000 unique genes. Genes were considered differentially expressed if they had a fold change greater than 1.5 and an altered P value of < .05 by the Benjamini-Hochberg false discovery rate calculation.
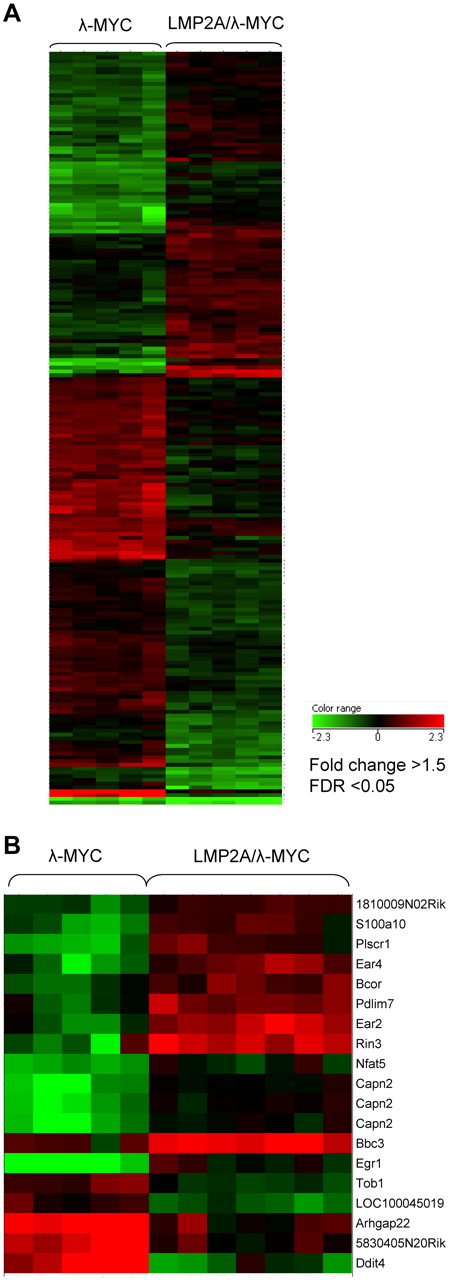
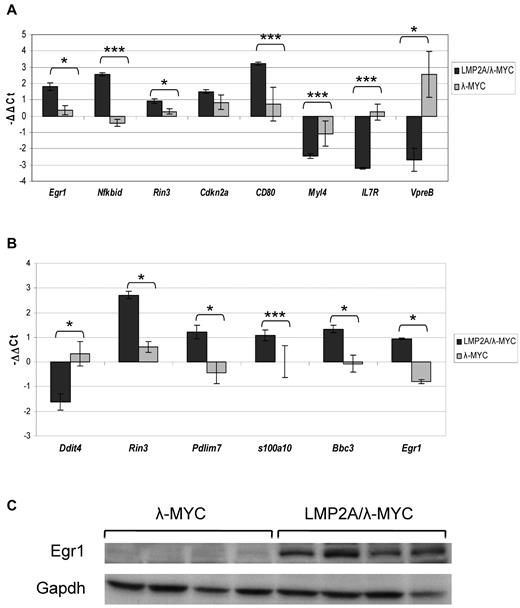
No significant differences in gene expression were measured in comparing B cells from LMP2A mice with wild-type mice. However, comparison of gene expression profiles of pretumor B cells from LMP2A/λ-MYC and λ-MYC mice revealed substantial differences. Figure 3A shows the heat map of 199 probe sequences that are differentially expressed by our statistical parameters. These 199 probes correspond to 187 unique genes, 77 of which are up-regulated in the LMP2A/λ-MYC pretumor B cells, whereas 110 are down-regulated in the LMP2A/λ-MYC pretumor B cells (relative to expression in λ-MYC B cells). The list of up-regulated genes, their fold change differences and P values can be found in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and the list of down-regulated genes in supplemental Table 2. To validate the differential expression of genes identified by microarray analysis, the expression level of a few of the genes in supplemental Tables 1 and 2 was examined by real-time RT-PCR. Quantitative PCR with primers specific for Egr1, Nfkbid, Rin3, and CD80 confirmed the statistically significant increase in expression of these genes in LMP2A/λ-MYC pretumor cells. Statistically significant decreased expression of Myl4, IL7R, and VpreB in LMP2A/λ-MYC pretumor cells was also confirmed (Figure 4A).
Differentially expressed probe sequences in tumor and pretumor B cells. Data analysis was performed using GeneSpringGX software. Probes were included if the fold change was greater than 1.5 and false discovery rate (adjusted P value) < .05. (A) A total of 199 probe sequences are differentially expressed in pretumor B cells. The 199 probes correspond to 187 unique genes, 77 are up-regulated in the LMP2A/λ-MYC samples, and 110 are down-regulated in the LMP2A/λ-MYC samples. (B) Nineteen probe sequences are differentially expressed in cervical lymph node tumor samples. The 19 probes correspond to 17 unique genes, 12 are up-regulated in the LMP2A/λ-MYC samples, and 5 are down-regulated in the LMP2A/λ-MYC samples.
Differentially expressed probe sequences in tumor and pretumor B cells. Data analysis was performed using GeneSpringGX software. Probes were included if the fold change was greater than 1.5 and false discovery rate (adjusted P value) < .05. (A) A total of 199 probe sequences are differentially expressed in pretumor B cells. The 199 probes correspond to 187 unique genes, 77 are up-regulated in the LMP2A/λ-MYC samples, and 110 are down-regulated in the LMP2A/λ-MYC samples. (B) Nineteen probe sequences are differentially expressed in cervical lymph node tumor samples. The 19 probes correspond to 17 unique genes, 12 are up-regulated in the LMP2A/λ-MYC samples, and 5 are down-regulated in the LMP2A/λ-MYC samples.
Validation of selected targets in pretumor and tumor B cells. RNA was isolated from purified B cells from 3-week-old λ-MYC and LMP2A/λ-MYC mice (A) or cervical lymph node tumor cells from tumor-burdened λ-MYC and LMP2A/λ-MYC mice (B) and converted to cDNA. Real-time RT-PCR analysis was performed using primers specific for the indicated target gene as well as the housekeeping gene Hprt. Each bar represents the average ΔΔCt for at least 3 different mice, and the ΔΔCt was calculated using one of the 3 individual λ-MYC mice as the reference sample to set the baseline for each gene. Error bars represent SEM. *P ≤ .05. ***P ≤ .01. (C) Immunoblot analysis shows Egr1 expression in tumor cells from LMP2A/λ-MYC mice and λ-MYC mice. Each lane represents a single tumor of the indicated genotype, and the data are representative of several immunoblot analyses.
Validation of selected targets in pretumor and tumor B cells. RNA was isolated from purified B cells from 3-week-old λ-MYC and LMP2A/λ-MYC mice (A) or cervical lymph node tumor cells from tumor-burdened λ-MYC and LMP2A/λ-MYC mice (B) and converted to cDNA. Real-time RT-PCR analysis was performed using primers specific for the indicated target gene as well as the housekeeping gene Hprt. Each bar represents the average ΔΔCt for at least 3 different mice, and the ΔΔCt was calculated using one of the 3 individual λ-MYC mice as the reference sample to set the baseline for each gene. Error bars represent SEM. *P ≤ .05. ***P ≤ .01. (C) Immunoblot analysis shows Egr1 expression in tumor cells from LMP2A/λ-MYC mice and λ-MYC mice. Each lane represents a single tumor of the indicated genotype, and the data are representative of several immunoblot analyses.
In contrast to our analysis of pretumor B cells, comparison of the gene expression profiles of tumor B cells from LMP2A/λ-MYC and λ-MYC mice showed that tumor cells from the two groups are transcriptionally quite similar. Only 19 probes met our statistical cut-off for differential expression (Figure 3B). These 19 probes correspond to 17 unique genes. Twelve genes are up-regulated in the LMP2A/λ-MYC tumor B cells, and 5 genes are down-regulated in the LMP2A/λ-MYC tumor B cells (Table 5). Again, we confirmed the differential expression of several targets by real-time RT-PCR. LMP2A/λ-MYC tumor cells showed statistically significant elevated expression of Rin3, Pdlim7, s100a10, Bbc3, and Egr1, whereas expression of Ddit4 was decreased compared with λ-MYC (Figure 4B). Increased expression of Egr1 protein was confirmed by immunoblot (Figure 4C).
Significantly up-regulated and down-regulated genes in LMP2A/λ-MYC tumors
| Symbol . | Definition . | Fold change . | P . | False discovery rate . |
|---|---|---|---|---|
| Up-regulated genes | ||||
| Egr1 | Early growth response 1 | 3.6304 | .0000135 | 0.0239 |
| Rin3 | Ras and Rab interactor 3 | 2.5029 | .000115 | 0.0430 |
| Ear2 | Eosinophil-associated, ribonuclease A family, member 2 | 2.2655 | .0000292 | 0.0330 |
| Ear4 | Eosinophil-associated, ribonuclease A family, member 4 | 2.0558 | .00013 | 0.0448 |
| Capn2 | Calpain 2 | 1.9949 | .000116 | 0.0430 |
| Plscr1 | Phospholipid scramblase 1 | 1.9808 | .000017 | 0.0249 |
| Bbc3 | BCL2 binding component 3 | 1.9007 | .0000921 | 0.0429 |
| Pdlim7 | PDZ and LIM domain 7 | 1.7375 | .0000884 | 0.0429 |
| S100a10 | S100 calcium binding protein A10 (calpactin) | 1.7371 | .000106 | 0.0429 |
| Bcor | Bcl6 interacting corepressor (Bcor), transcript variant a | 1.6400 | .000142 | 0.0469 |
| Nfat5 | Nuclear factor of activated T-cells 5 | 1.5846 | .0000906 | 0.0429 |
| 1810009N02Rik | RIKEN cDNA 1810009N02 gene | 1.5233 | .0000069 | 0.0239 |
| Down-regulated genes | ||||
| Tob1 | Transducer of ErbB-2.1 | −1.5070 | .0000857 | 0.0429 |
| LOC100045019 | Predicted: Mus musculus similar to tubulin, gamma 2 | −1.5685 | .0000481 | 0.0378 |
| 5830405N20Rik | RIKEN cDNA 5830405N20 gene | −1.9015 | .000104 | 0.0429 |
| Arhgap22 | Rho GTPase activating protein 22 | −2.0540 | .0000303 | 0.0330 |
| Ddit4 | DNA-damage-inducible transcript 4 | −3.1498 | .0000681 | 0.0429 |
| Symbol . | Definition . | Fold change . | P . | False discovery rate . |
|---|---|---|---|---|
| Up-regulated genes | ||||
| Egr1 | Early growth response 1 | 3.6304 | .0000135 | 0.0239 |
| Rin3 | Ras and Rab interactor 3 | 2.5029 | .000115 | 0.0430 |
| Ear2 | Eosinophil-associated, ribonuclease A family, member 2 | 2.2655 | .0000292 | 0.0330 |
| Ear4 | Eosinophil-associated, ribonuclease A family, member 4 | 2.0558 | .00013 | 0.0448 |
| Capn2 | Calpain 2 | 1.9949 | .000116 | 0.0430 |
| Plscr1 | Phospholipid scramblase 1 | 1.9808 | .000017 | 0.0249 |
| Bbc3 | BCL2 binding component 3 | 1.9007 | .0000921 | 0.0429 |
| Pdlim7 | PDZ and LIM domain 7 | 1.7375 | .0000884 | 0.0429 |
| S100a10 | S100 calcium binding protein A10 (calpactin) | 1.7371 | .000106 | 0.0429 |
| Bcor | Bcl6 interacting corepressor (Bcor), transcript variant a | 1.6400 | .000142 | 0.0469 |
| Nfat5 | Nuclear factor of activated T-cells 5 | 1.5846 | .0000906 | 0.0429 |
| 1810009N02Rik | RIKEN cDNA 1810009N02 gene | 1.5233 | .0000069 | 0.0239 |
| Down-regulated genes | ||||
| Tob1 | Transducer of ErbB-2.1 | −1.5070 | .0000857 | 0.0429 |
| LOC100045019 | Predicted: Mus musculus similar to tubulin, gamma 2 | −1.5685 | .0000481 | 0.0378 |
| 5830405N20Rik | RIKEN cDNA 5830405N20 gene | −1.9015 | .000104 | 0.0429 |
| Arhgap22 | Rho GTPase activating protein 22 | −2.0540 | .0000303 | 0.0330 |
| Ddit4 | DNA-damage-inducible transcript 4 | −3.1498 | .0000681 | 0.0429 |
The relatively short list of differentially regulated genes identified in comparing the gene expression profiles of LMP2A/λ-MYC and λ-MYC tumor B cells is surprising because of the fairly dramatic phenotypes observed in the LMP2A/λ-MYC mice, such as acceleration of tumor onset and bypass of p53 inactivation.20 The more slowly arising λ-MYC tumors may be more heterogeneous than LMP2A/λ-MYC tumors, which could result in more variation among their gene expression profiles. Increased heterogeneity of λ-MYC tumors could contribute to the small number of differentially expressed genes identified in the tumor comparison. However, clear subgroups within λ-MYC tumor samples could not be delineated, even if the samples were separated by IgM expression or p53 status, or when additional λ-MYC tumor samples were included. One interpretation of the small number of target genes identified in comparing LMP2A/λ-MYC and λ-MYC tumors is that these genes are particularly important for the effects of LMP2A in the presence of deregulated MYC.
Egr1 in LMP2A/λ-MYC tumors
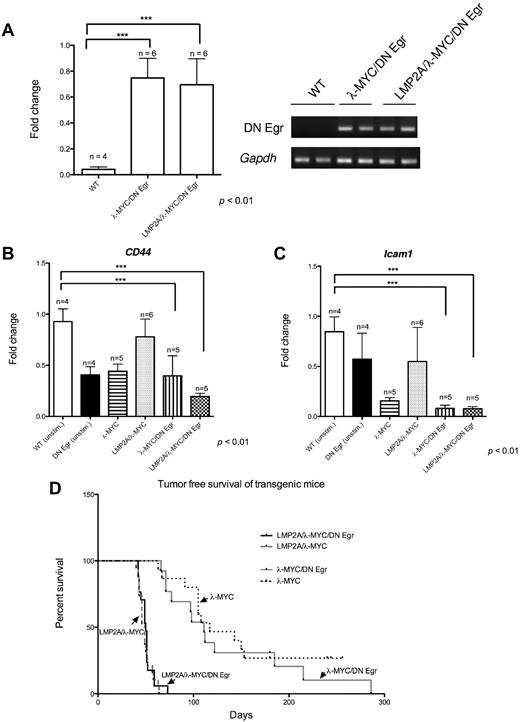
The gene with the largest fold change value in comparing gene expression in LMP2A/λ-MYC and λ-MYC tumor B cells is early growth response gene-1 (Egr1, Figure 4B; Table 5). Egr1 expression was also increased at the protein level in LMP2A/λ-MYC tumors (Figure 4C), and in LMP2A/λ-MYC pretumor B cells compared with λ-MYC pretumor B cells (Figure 4A; supplemental Table 1). We were particularly interested in this gene for several reasons. First, Egr1 is known to be differentially expressed in some types of human cancer. For example, increased Egr1 expression is commonly observed in prostate cancer cells,26,27 and Egr1 is important for progression to malignancy in prostate cancer cell lines28-30 and mouse models.31 The Egr1 gene encodes a transcription factor expressed in many cell types whose function depends on cell type and context; Egr1 is known to function in cell differentiation, growth promotion and growth suppression, and apoptosis.32-34 Egr1 belongs to the immediate early gene family and is rapidly induced in response to various stimuli, including growth factors, cytokines, and irradiation, varying by cell type.32,35-37 In B cells, Egr1 is induced by BCR signaling.24,33,38 Given that LMP2A is a signaling molecule that is functionally similar to the BCR,10,11,22 we hypothesized that induction of Egr1 in LMP2A/λ-MYC mice may contribute to their accelerated tumorigenesis.20,21 To test the functional importance of Egr1 in our transgenic model, we used a transgenic mouse expressing a DN Egr in B cells.24 The DN Egr used in our studies consists of a truncated version of the Egr1 protein, containing only the DNA binding domain, which is expressed from an Ig promoter, targeting expression to B cells. The dominant negative has been shown to inhibit the functions of all Egr family members and subtly alter B-cell differentiation and responses to BCR ligation.24
We generated triple transgenic mice expressing LMP2A, MYC, and DN Egr in B cells. LMP2A/λ-MYC/DN Egr mice and λ-MYC/DN Egr mice both develop lymph node tumors, and these tumors contain similar levels of DN Egr mRNA (Figure 5A). To verify function of the DN Egr transgene, we examined expression of two established transcriptional targets of Egr1 in B cells, CD44 and Icam-1.39,40 Consistent with previous studies,24 unstimulated B cells from DN Egr transgenic mice have reduced expression of CD44 as well as Icam-1 relative to wild-type B cells (Figure 5B-C). Levels of both CD44 and Icam-1 were slightly higher, though not statistically significant, in tumor cells from LMP2A/λ-MYC relative to λ-MYC tumors. These results are consistent with our demonstration of Egr1 up-regulation in LMP2A/λ-MYC tumors (Figures 3 and 4). Furthermore, tumor cells from both λ-MYC/DN Egr and LMP2A/λ-MYC/DN Egr mice show significantly down-regulated expression of CD44 and Icam-1 relative to wild-type B cells. These data suggest that the DN Egr transgene is functional in the LMP2A/λ-MYC and λ-MYC tumor models, inhibiting transcriptional targets of endogenous Egr1. To assess the role of Egr1 in acceleration of tumor onset by LMP2A, we monitored cohorts of transgenic mice. Tumor mortality in the LMP2A/λ-MYC/DN Egr mice was no different from the tumor mortality we observed in LMP2A/λ-MYC mice (Figure 5D). Similarly, tumor onset in λ-MYC/DN Egr transgenic mice was not different from λ-MYC mice (Figure 5D). Taken together, these results demonstrate that functional inhibition of Egr1 does not affect tumorigenesis in LMP2A/λ-MYC mice, suggesting that induction of Egr1 is not responsible for acceleration of tumor onset by LMP2A.
Generation of LMP2A/λ-MYC/DN Egr transgenic mice. RNA was isolated from cervical lymph node tumor cells from tumor-burdened transgenic mice of the indicated genotypes or purified B cells from wild-type and DN Egr transgenic mice. Real-time RT-PCR analysis was performed using primers specific for DN Egr (A), CD44 (B), and Icam-1 (C) as well as the housekeeping gene Gapdh. The inset in panel A represents RT-PCR products electrophoresed on 2% agarose gel from 1 experiment. unstim. indicates unstimulated B cells from wild-type and DN Egr mice. All RT-PCR data represent the mean ± SE from 3 independent experiments. The differences in gene expression of all groups were analyzed by 1-way ANOVA and Dunnett multiple comparison tests using wild-type as a control group. ***P < .01. (D) Kaplan-Meier curve shows percentage survival of each transgenic line, LMP2A/λ-MYC/DN Egr: n = 17, LMP2A/λ-MYC: n = 19, λ-MYC/DN Egr: n = 14, λ-MYC: n = 16. Mice were sacrificed when cervical lymph node tumors were observed, at which point the animals could generally survive for a maximum of 7 days.
Generation of LMP2A/λ-MYC/DN Egr transgenic mice. RNA was isolated from cervical lymph node tumor cells from tumor-burdened transgenic mice of the indicated genotypes or purified B cells from wild-type and DN Egr transgenic mice. Real-time RT-PCR analysis was performed using primers specific for DN Egr (A), CD44 (B), and Icam-1 (C) as well as the housekeeping gene Gapdh. The inset in panel A represents RT-PCR products electrophoresed on 2% agarose gel from 1 experiment. unstim. indicates unstimulated B cells from wild-type and DN Egr mice. All RT-PCR data represent the mean ± SE from 3 independent experiments. The differences in gene expression of all groups were analyzed by 1-way ANOVA and Dunnett multiple comparison tests using wild-type as a control group. ***P < .01. (D) Kaplan-Meier curve shows percentage survival of each transgenic line, LMP2A/λ-MYC/DN Egr: n = 17, LMP2A/λ-MYC: n = 19, λ-MYC/DN Egr: n = 14, λ-MYC: n = 16. Mice were sacrificed when cervical lymph node tumors were observed, at which point the animals could generally survive for a maximum of 7 days.
Discussion
Gene expression profiling is a powerful tool that can be used to categorize, clarify the origins, and potentially even identify the underlying causes of malignancies. We analyzed total gene expression in a transgenic mouse model for EBV-associated BL. There are some major phenotypic differences in tumors from LMP2A/λ-MYC and λ-MYC mice. The tumors differ in IgM expression (Figure 2A) and p53 status,20 each of which could heavily influence gene expression. However, our results show that gene expression in LMP2A/λ-MYC and λ-MYC tumors is surprisingly similar. Some of the differentially expressed genes are consistent with known characteristics of LMP2A/λ-MYC tumors, supporting the validity of our analysis. For example, Bbc3, which encodes Puma, a proapoptotic p53 target gene, showed increased expression in LMP2A/λ-MYC tumors, consistent with a previous report.20 Ddit4 or DNA damage inducible transcript 4 was significantly down-regulated in LMP2A/λ-MYC tumors (Figures 3 and 4; Table 5). Ddit4 is also known as REDD1 and functions to modulate an important cell growth pathway by negatively regulating mammalian target of rapamycin (mTOR). In a tumor transfer model, LMP2A/λ-MYC tumors were more dependent on the mTOR pathway and more effectively treated with rapamycin relative to λ-MYC tumors.41
One of the most interesting differentially expressed genes identified in our studies was the transcription factor Egr1. The importance of Egr1 in regulating expression of many genes involved in cell survival, growth, and differentiation32-34 suggested to us that Egr1 may be important for the ability of LMP2A to accelerate tumor onset in the presence of deregulated MYC. That Egr1 induction is downstream of BCR signaling24,33,38 and LMP2A can be described as a BCR signaling “mimic” leant further support to our hypothesis. However, Egr1 function, at least the amount that could be blocked by expression of a dominant negative Egr,24 does not appear to be required for tumor acceleration by LMP2A (Figure 5).
In contrast to LMP2A/λ-MYC and λ-MYC tumors, we see a much greater difference in gene expression in LMP2A/λ-MYC and λ-MYC pretumor B cells (Figure 6), with a 10-fold increase in the number of differentially expressed genes. The increase in differential gene expression in pretumor mice is probably largely associated with the phenotypic differences between LMP2A/λ-MYC and λ-MYC mice that can be observed at early ages. Before tumors arise in these mice, we see differences in B-cell development (Figure 1A), spleen size,21 splenic architecture (Figure 1B), and cell cycle profiles (Figure 1C).21 Although B-cell development is not altered in the Tg6-LMP2A transgenic line used in these studies23 (Figure 1) and microarray analysis comparing the Tg6-LMP2A transgenic line to wild-type mice showed no differences in gene expression in the absence of BCR activation,42 here we show that, in the presence of overexpressed MYC, even low levels of LMP2A expression from the Tg6 transgene23 have a great impact on B-cell development.
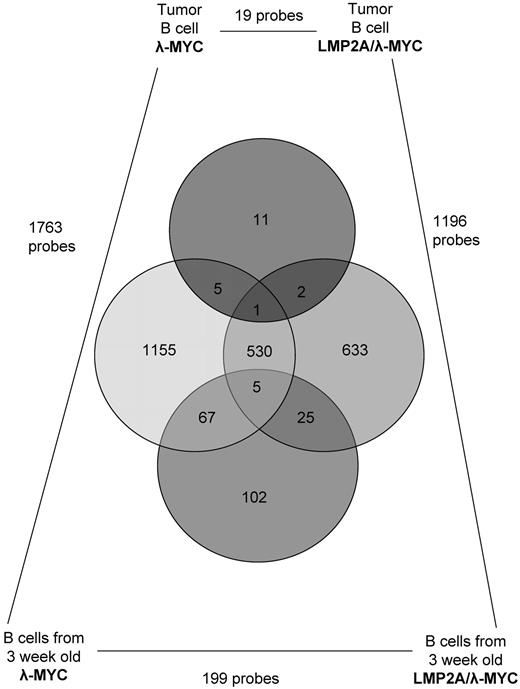
Overlapping gene lists in gene expression comparisons in tumor and pretumor B cells. Nineteen probes are significantly differentially expressed when comparing LMP2A/λ-MYC and λ-MYC tumor B cells. In contrast, 199 probes are differentially expressed when comparing LMP2A/λ-MYC and λ-MYC pretumor B cells. A total of 1196 probes and 1763 probes are differentially expressed when comparing pretumor B cells with tumor B cells in LMP2A/λ-MYC and λ-MYC, respectively. Each circle in the Venn diagram represents the probes that are differentially expressed in comparing the samples indicated in the corners. The probes that are differentially expressed in multiple comparisons are shown by the overlapping circles.
Overlapping gene lists in gene expression comparisons in tumor and pretumor B cells. Nineteen probes are significantly differentially expressed when comparing LMP2A/λ-MYC and λ-MYC tumor B cells. In contrast, 199 probes are differentially expressed when comparing LMP2A/λ-MYC and λ-MYC pretumor B cells. A total of 1196 probes and 1763 probes are differentially expressed when comparing pretumor B cells with tumor B cells in LMP2A/λ-MYC and λ-MYC, respectively. Each circle in the Venn diagram represents the probes that are differentially expressed in comparing the samples indicated in the corners. The probes that are differentially expressed in multiple comparisons are shown by the overlapping circles.
A closer look at some of the individual genes that are differentially expressed in pretumor B cells demonstrates the influence of altered B-cell development on gene expression. The gene with the highest fold-change is Vpreb1 (supplemental Table 2; Figure 4), which is down-regulated in LMP2A/λ-MYC pretumor B cells. Vpreb1 is a component of the pre-BCR, previously shown to be altered by LMP2A in bone marrow cells.15 Dntt, terminal deoxynucleotidyltransferase, was also reduced in LMP2A/λ-MYC pretumor B cells (supplemental Table 2). Expression of both of these developmentally regulated targets was previously shown to be altered by LMP2A in the TgE line43 but was not changed in the Tg6-LMP2A line.42 The high amount of surface B220 and CD43 (Figure 1A) coupled with the absence of surface IgM (Figure 1A) and reduced levels of VpreB1 and Dntt mRNA suggest that B cells in the LMP2A/λ-MYC mice may be arrested at the pro-B to pre-B stage of development. Interestingly, mRNA levels of the IL-7 receptor are also reduced in LMP2A/λ-MYC pretumor B cells (supplemental Table 2; Figure 4). Expression of IL-7R is also developmentally regulated as IL-7 receptor signaling is required for survival of early B-cell populations in the bone marrow, and pro-B cells generally express high levels of IL-7R. In our mice, the combination of the survival pathways activated by LMP2A13,22 and proliferative pathways activated by MYC may preclude the requirement for signaling through either the preBCR or IL-7R during early stages of B-cell development.
The pretumor B cells also have altered expression of some signaling molecules, including Pik3cd, Inpp5d, Plcd3, and Mapk12 (supplemental Tables 1-2). LMP2A has been shown to activate numerous signaling pathways12-15 that are generally regulated through post-translational protein modification. In light of the striking transcriptional similarity between LMP2A/λ-MYC and λ-MYC tumors, further investigation of the differences between LMP2A/λ-MYC and λ-MYC tumors at the protein or post-translational level is needed. Such studies may be helpful in identifying cellular proteins or pathways that are required for the role of LMP2A in tumorigenesis and lead to identification of novel drug targets for EBV-associated malignancies.
We can use our gene expression analysis of LMP2A/λ-MYC and λ-MYC tumors to enhance our overall understanding of the role of EBV in BL. The transition from a normal B cell to a tumor cell requires multiple cellular changes. In human BL, after the MYC translocation, mutation in p53 is a common genetic change found in tumor biopsies.44,45 In our study, we see massive changes in gene expression when comparing LMP2A/λ-MYC pretumor B cells with LMP2A/λ-MYC tumor B cells (1196 probes) or λ-MYC pretumor B cells to λ-MYC tumor B cells (1763 probes; Figure 6). In MYC transgenic mice that express LMP2A, tumor onset is faster,20,21 and we do not find mutations in p53.20 In addition, fewer changes in gene expression occur in the transition from a pretumor B cell to a tumor B cell (Figure 6). However, once tumors develop in LMP2A/λ-MYC or λ-MYC mice, gene expression in B cells is only minimally different. The similarity in gene expression profiles supports our proposed model for the role of EBV in BL.46 Our model purports that LMP2A is most important early in tumorigenesis by promoting survival in the presence of deregulated MYC while additional cellular changes are acquired. These same changes may also occur in non–EBV-associated BL, but the presence of LMP2A increases the likelihood that an additional “hit” will occur in a cell that overexpresses MYC. After tumor progression, dependence on LMP2A decreases and EBV+ and EBV− tumor cells lack significant transcriptional differences. The similarity between EBV-associated and non–EBV-associated BL has resulted in difficulty in understanding the function(s) of EBV in BL development. Here we have shown that the LMP2A/λ-MYC model reflects this puzzle, revealing nearly identical gene expression profiles in LMP2A+ and LMP2A− tumors. These data highlight the utility of the mouse model in understanding human BL pathogenesis and developing drug targets for human disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nadereh Jafari and the Genomics Core Facility for assistance with the microarrays, Simon Lin and Gilbert Feng at the Bioinformatics Core Facility for their support of the statistical analysis, and members of R.L.'s laboratory for help in the completion of these studies.
This work was supported by the Northwestern University Mouse Histology and Phenotyping Laboratory and the National Cancer Institute (Cancer Center Support grant CA060553) and the Northwestern University Interdepartmental ImmunoBiology Flow Cytometry Core Facility. R.L. is a John Edward Porter Professor in Biomedical Research and is supported by the National Cancer Institute (Public Health Service grants CA 133063 and CA73507). K.T.B. was supported in part by the Carcinogenesis Training Program (T32CA009560). S.B. was supported by the National Cancer Institute (grant 5P01CA092372).
National Institutes of Health
Authorship
Contribution: K.T.B. and K.F. designed and performed research, analyzed data, and wrote the manuscript; S.B. contributed vital reagents; and R.L. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard Longnecker, Northwestern University, 303 East Chicago Ave, Chicago, IL 60614; e-mail: r-longnecker@northwestern.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal