Abstract
IL-15 promotes activation and maintenance of natural killer (NK) and CD8+ T effector memory (TEM) cells, making it a potential immunotherapeutic agent for the treatment of cancer and immunodeficiency states. Here we report the immunologic effects of 3 different IL-15 dosing strategies in Rhesus macaques. IL-15 at a dose of 20 μg/kg/d administered by continuous intravenous infusion for 10 days resulted in a massive (100-fold) expansion of CD8+ TEM cells in the peripheral blood. In contrast, the administration of 20-40 μg/kg/d of IL-15 by subcutaneous injection resulted in a more modest (10-fold) expansion of CD8+ TEM cells. NK expansion was similar in both the continuous intravenous and daily subcutaneous treatment groups. The observation that IL-15 administered by continuous intravenous infusion is able to induce markedly greater expansions of CD8+ TEM cells than the same dose administered by other routes may have important implications for clinical development of this cytokine.
Introduction
IL-15 is a member of the common γ-chain family of cytokines that shares functional activities with IL-2. The main nonredundant role of IL-15 in immune homeostasis appears to be in the maintenance of long-lasting T-cell immunity by supporting the proliferation and survival of memory CD8+ T cells.1 Because of the critical role of IL-15 in maintaining CD8+ T-cell memory responses, it has been viewed as a potential immunotherapeutic agent for augmenting endogenous or adoptively transferred T-cell immunity in humans with diseases such as chronic HIV infection or certain forms of cancer.1-3 Preclinical studies of IL-15 administered to Rhesus macaques (RMs) have given variable results with regards to in vivo expansion of the CD8+ T-cell memory pool. At doses of 10-20 μg/kg given subcutaneously twice a week, minimal (1- to 3-fold) expansion of CD8+ T effector memory (CD8+ TEM) has been observed.4-6 In contrast, IL-15 in this dose range given as a single daily intravenous injection resulted in somewhat greater expansions (∼ 6-fold) of CD8+ TEM.7 In an attempt to explore the immunologic effects of different IL-15 dosing strategies, we treated RMs with IL-15 administered either by continuous intravenous infusion (CIV) or subcutaneous (SC) injection.
Methods
Recombinant human IL-15 was produced under current good manufacturing practice conditions in an Escherichia coli system as previously described.8 RMs were assigned to 5 treatment groups. Group 1 (3 animals) served as a control and received 5% dextrose by CIV. Group 2 (6 animals) received 20 μg/kg/d of IL-15 for 10 days by CIV. Group 3 (3 animals) received 20 μg/kg of IL-15 by SC injection twice a week for 2 weeks. Groups 4 and 5 (3 animals each) received 20 μg/kg or 40 μg/kg/d of IL-15 by daily SC injection for 10 days. Animals in groups 1 and 2 had vascular access ports surgically implanted and received IL-15 diluted in 5% dextrose or 5% dextrose alone (group 1) via constant infusion pumps. Animals in group 2 were treated with 2 different lots of IL-15 (3 animals each). There was no difference in the in vitro potency or in vivo effects of the different lots of IL-15 and these groups were combined for purposes of data analysis. The animals in the SC groups all received the same lot of IL-15. All animal experiments were approved by the Institutional Animal Care and Use Committee of Avanza Laboratories. Routine clinical chemistry and hematology parameters were measured before and 7, 10, 17, 24, and 40 days after the initiation of each treatment period.
Immunophenotypic analysis was performed on whole blood before initiation of each treatment period and 10, 17, 24, and 40 days after initiation of each treatment period. T, B, and NK subsets were identified on cells gated by side-scatter/CD45 expression with B cells identified as CD3−/CD20+ and NK as CD3−/CD16+. With the exception of anti-CD8 antibody (RPA-T8; Becton Dickinson), effector/memory subsets were identified by CD95/CD28 expression using the methods, gating strategies, and antibodies previously described6,9 on cells gated using light scatter plus CD3/CD4 or CD3/CD8, respectively. Serum levels of IL-15 and anti–IL-15 antibodies were measured as previously described.8
Results and discussion
Overall, the administration of IL-15 was well tolerated with toxicities similar to those previously described with daily intravenous dosing.8 There were no apparent long-term effects on clinical observations, body weights, or qualitative food consumption. One animal in the CIV group was unexpectedly found dead on study day 72. Routine hematology and chemistry studies done 5 days before death were normal. Necropsy did not reveal the cause of death.
Animals in the CIV cohort exhibited moderate increases (4-7 times control cohort values) in aspartate aminotransferase and alanine aminotransferase at study days 7 and 10. These elevations were transient and returned to baseline between days 24 and 40. A transient decrease in serum albumin (mean value 3.0 gm/dL) was noted at day 10 in the CIV cohort; values returned to normal by day 24. Animals in the daily SC cohorts exhibited mild-moderate increases (2-3 times control cohort values) in ALT/AST at day 10. In addition, animals in the 40 μg/kg/d SC cohort exhibited hyponatremia (mean serum sodium 127 mmol/L) at day 10. These abnormalities were transient and resolved by day 24. No significant laboratory abnormalities were seen in the control or twice-weekly SC cohorts.
Two animals in the CIV cohort and 1 animal in the 40 μg/kg/d SC cohort developed transient neutropenia (ANC < 500 cells/μL) on day 10 of the treatment period, that resolved within 7 days after completion of treatment. With the exception of lymphocytosis (see next paragraph), no other clinically significant abnormalities of hematology or chemistry parameters were observed during IL-15 treatment in any of the cohorts.
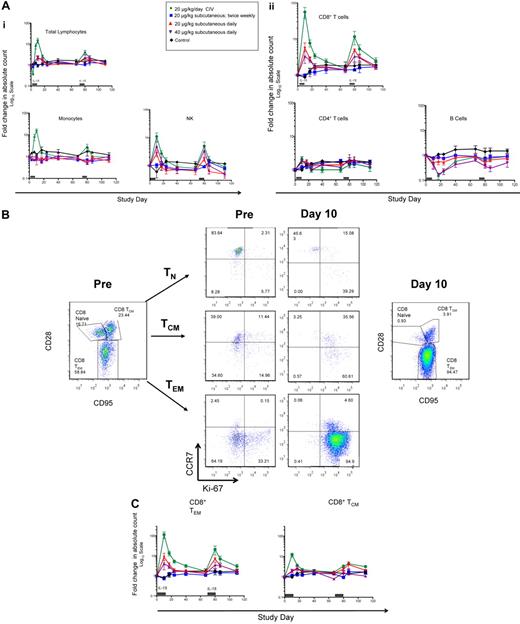
The most dramatic hematologic effects were seen in animals receiving IL-15 by CIV in which a transient lymphopenia at treatment day 3 was followed by a dramatic lymphocytosis (Figure 1A). The lymphocytosis peaked on the last day of the IL-15 infusion (day 10) with a median lymphocyte count of 79 523 cells/μL (interquartile range 51 278-118 150). An approximately 15-fold increase in monocytes was also observed in the CIV group (Figure 1A). The lymphocytosis was predominately because of an approximately 100-fold expansion of CD8+ T cells exhibiting a TEM phenotype (Figure 1B-C) with a median count of 52 885 CD8+ TEM cells/μL (interquartile range 28 574-80 140). A lesser (approximately 10-fold) expansion of CD8+ T central memory (TCM) and NK cells was also observed (Figure 1) along with a modest (4- to 5-fold) increase in CD4+ TCM and TEM cells (data not shown). A transient 5- to 6-fold decrease in B cells was observed at study day 17 (Figure 1A). There were no significant changes in naive CD4+ or CD8+ T-cell numbers. Animals in the daily SC cohorts exhibited a similar pattern of response; however, the magnitude CD8+ TEM expansion was approximately 10-fold less (Figure 1C) than that observed in the CIV group. No significant expansion of monocytes was observed in the daily SC cohorts (Figure 1A). Animals who received twice-weekly subcutaneous injections of IL-15 or sham CIV infusion did not exhibit any significant increase above pretreatment levels of monocytes, NK cells, B cells, CD4+, or CD8+ lymphocyte subsets (Figure 1 and data not shown).
IL-15 administered by continuous intravenous infusion (CIV) results in 100-fold expansion of the CD8+ T effector memory population. IL-15 was administered for 10 days using 4 different dosing strategies (see panel A description below). The control group received 5% dextrose by CIV for 10 days. (A) Effect of IL-15 administration on total lymphocyte count, monocyte count, and lymphocyte subsets. Each point corresponds to the mean ± SEM and indicates the fold change in the absolute count relative to pretreatment (day −4). Gray bars above x-axis represent duration of IL-15 administration. (B) Representative example of CD8+ memory subset expansion in a RMs receiving 20 μg/kg/d of IL-15 by CIV. The left panel shows the pretreatment distribution of CD3+CD8+ naive (TN, CD28+ CD95−), central memory (TCM, CD28+ CD95+), and effector memory (TEM, CD28− CD 95+) populations. The right panel shows the distribution of CD3+CD8+ TN, TCM, and TEM populations after 10 days of IL-15 treatment. The center panels show pre- and day-10 expression of Ki67 and CCR7 on CD3+CD8+ TN, TCM, and TEM gated populations. (C) Dynamics of CD8+ memory subset expansion by different IL-15 dosing strategies. Data from the different dose groups are shown as in panel A.
IL-15 administered by continuous intravenous infusion (CIV) results in 100-fold expansion of the CD8+ T effector memory population. IL-15 was administered for 10 days using 4 different dosing strategies (see panel A description below). The control group received 5% dextrose by CIV for 10 days. (A) Effect of IL-15 administration on total lymphocyte count, monocyte count, and lymphocyte subsets. Each point corresponds to the mean ± SEM and indicates the fold change in the absolute count relative to pretreatment (day −4). Gray bars above x-axis represent duration of IL-15 administration. (B) Representative example of CD8+ memory subset expansion in a RMs receiving 20 μg/kg/d of IL-15 by CIV. The left panel shows the pretreatment distribution of CD3+CD8+ naive (TN, CD28+ CD95−), central memory (TCM, CD28+ CD95+), and effector memory (TEM, CD28− CD 95+) populations. The right panel shows the distribution of CD3+CD8+ TN, TCM, and TEM populations after 10 days of IL-15 treatment. The center panels show pre- and day-10 expression of Ki67 and CCR7 on CD3+CD8+ TN, TCM, and TEM gated populations. (C) Dynamics of CD8+ memory subset expansion by different IL-15 dosing strategies. Data from the different dose groups are shown as in panel A.
After an 8-week washout period, a second treatment course was administered. When compared with the first treatment period, animals in the CIV group exhibited a smaller, yet still impressive (∼ 20-fold) expansion of CD8+ TEM cells (Figure 1C). Responses in the daily subcutaneous cohorts were similar to those observed in the initial course (Figure 1C).
To further evaluate the longitudinal effect of multiple treatment courses of IL-15 administered by CIV, a third 10-day treatment course was administered to the 5 surviving animals in the CIV cohort. The third CIV treatment was given 9 months after the second and again resulted in an approximately 20-fold expansions of CD8+ TEM cells similar to the second course of treatment (data not shown).
To determine if the diminished responses observed in the CIV cohort during the second and third treatment periods were because of immunogenicity of the human IL-15 preparation, serum was analyzed for anti–IL-15 antibodies using a 2-arm capture ELISA procedure (limit of detection of 350 ng/mL). All pretreatment samples tested negative for anti–IL-15 antibody. At the end of the first IL-15 treatment period 1 animal in the CIV group had detectable anti–IL-15 antibody; by the third treatment period, 3 of the 5 animals tested in the CIV group had detectable antibody with levels ranging from 669-5449 ng/mL. No animals in the control or subcutaneous groups developed anti–IL-15 antibody.
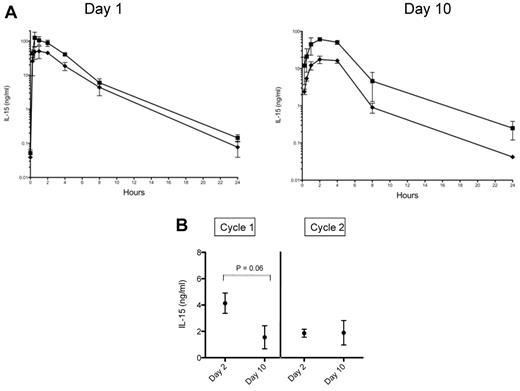
In a previous study we observed that 20 μg/kg of IL-15 given by intravenous bolus injection resulted in a Cmax of 702 ± 280 ng/mL and a terminal half-time of 1.1 hours with levels becoming undetectable by 24 hours.8 Animals in the 20 and 40 μg/kg/d SC cohorts of the present study had Cmax of 50 ± 19 ng/mL and 105 ± 29 ng/mL, respectively, after the first injection and 17 ± 4 ng/mL and 61 ± 2 ng/mL on day 10 of treatment (Figure 2A). In contrast, serum IL-15 levels obtained on days 2 and 10 in the CIV cohort were in the 2-4 ng/mL range (Figure 2B).
Pharmacokinetics of IL-15 when administered by continuous intravenous infusion versus subcutaneous dosing. (A) Serum levels of IL-15 were obtained at 0.25, 0.5, 1, 2, 4, 8, and 24 hours after dose on treatment days 1 and 10 in animals receiving 20 μg/kg/ (♦) or 40 μg/kg/d (■) of IL-15 by SC injection. Each point corresponds to the mean ± SD for 3 animals. (B) Serum IL-15 levels were measured on days 2 and 10 in RMs receiving 20 μg/kg/d of IL-15 given by CIV. Levels were measured during 2 treatment cycles given 8 weeks apart. Each point corresponds to the mean ± SD. For day 2 versus day 10 in cycle 1, P = .06 by Wilcoxon rank test.
Pharmacokinetics of IL-15 when administered by continuous intravenous infusion versus subcutaneous dosing. (A) Serum levels of IL-15 were obtained at 0.25, 0.5, 1, 2, 4, 8, and 24 hours after dose on treatment days 1 and 10 in animals receiving 20 μg/kg/ (♦) or 40 μg/kg/d (■) of IL-15 by SC injection. Each point corresponds to the mean ± SD for 3 animals. (B) Serum IL-15 levels were measured on days 2 and 10 in RMs receiving 20 μg/kg/d of IL-15 given by CIV. Levels were measured during 2 treatment cycles given 8 weeks apart. Each point corresponds to the mean ± SD. For day 2 versus day 10 in cycle 1, P = .06 by Wilcoxon rank test.
The current study describes a novel aspect of IL-15 pharmacodynamics that may have important implications for clinical development. We demonstrate that the administration of IL-15 at a dose of 20 μg/kg/d by CIV results in sustained low-serum levels of IL-15 and much larger expansion of CD8+ TEM cells than occurs when the same dose is given by daily intravenous injection,7 daily subcutaneous injection, or twice-weekly subcutaneous injection. The diminished expansion of CD8+ TEM cells seen with repeated courses of IL-15 given by CIV may be due to the development of neutralizing anti–IL-15 antibodies as has been previously described with the use of human IL-15 in RMs.10
The CIV route of administration resulted in sustained low-serum levels of IL-15 sufficient to act through the high-affinity 15Rα-β/γ receptor complex alone. It is thus likely that the massive expansion of CD8+ TEM cells observed when IL-15 is administered by CIV results from continuous stimulation through the high-affinity IL-15Rα-β/γ receptor complex via either trans11,12 or cis presentation; the latter mechanism having been recently demonstrated to occur in vitro with human but not murine CD8+ T cells.13 As with our previous study, the expanded cell populations declined to baseline after IL-15 was stopped, suggesting migration to extra-lymphoid sites.7 Our results indicate that IL-15 administered by CIV has the potential to induce much larger expansion of CD8+ TEM cells in humans than other dosing strategies. Based on the toxicity profile and immunologic effects of CIV dosing in RMs, strong consideration should be given to including this dosing strategy in future clinical trials of IL-15.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported by the intramural programs of the National Cancer Institute and the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: M.C.S. and H.C.L. conceived and supervised the study, analyzed the data, and wrote the paper; W.C.K. performed the experiments; K.J.E. provided veterinary support and supervised the study; J.L.Y. and S.P.C. produced the IL-15; and T.A.W. analyzed the data and supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael C. Sneller, MD, National Institutes of Health, 10 Center Dr, MSC 1763, Bethesda, MD 20892; e-mail: msneller@niaid.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal