In this issue of Blood, Hegde et al show that a novel and naturally produced eicosapentaenoic acid–derived cyclopentenone prostaglandin has antileukemic activity.1 Δ12-PGJ3–mediated apoptosis of leukemic stem cells is mediated at least in part by ATM-p53 signaling.
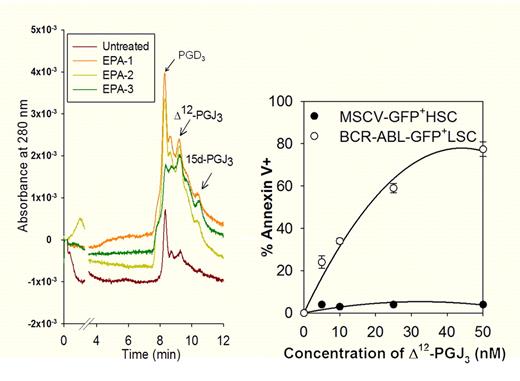
Antileukemic activity of eicosapentaenoic acid (EPA)–derived cyclopentenone prostaglandins. Left panel shows a liquid chromatography-UV trace of EPA-derived metabolites endogenously produced by macrophages. Right panel demonstrates the selective antileukemic effect of Δ12-PGJ3 on BCR-ABL–transformed leukemic stem cells.
Antileukemic activity of eicosapentaenoic acid (EPA)–derived cyclopentenone prostaglandins. Left panel shows a liquid chromatography-UV trace of EPA-derived metabolites endogenously produced by macrophages. Right panel demonstrates the selective antileukemic effect of Δ12-PGJ3 on BCR-ABL–transformed leukemic stem cells.
The salutory effects of fish oil ω-3 polyunsaturated fatty acids (n-3 PUFAs) include reduced inflammatory and cardiovascular disease and prevention of cancer.2,3 Apart from the role of n-3 PUFAs in disease prevention, recent studies suggest that n-3 PUFA metabolites may have a place in cancer treatment. In this regard, the arachidonic acid–derived prostaglandin, 15 d-PGJ2, has been reported to exhibit antileukemic activity.4 Hegde et al show that n-3 PUFA metabolites, endogenously produced by macrophages treated with eicosapentaenoic acid, have activity against leukemia stem cells at nanomolar concentrations.1 Leukemia stem cells are notoriously refractory to conventional drugs; consequently, their eradication is an important unrealized therapeutic goal. The current study offers hope for the establishment of a new class of drugs with antileukemic activity.
The investigators used 2 murine models of leukemia with well-defined leukemia stem cell populations. The first model employs expression of the fusion oncoprotein BCR-ABL in murine hematopoietic cells.5 BCR-ABL virus-infected Kit+Sca1+Lin− cells are exquisitely sensitive to Δ12-PGJ3–induced apoptosis. By contrast, treatment of control hematopoietic stem and progenitor cells has no effect. The second model employs Friend erythroleukemia virus. Friend virus contains an oncoprotein in the form of a mutant envelope protein, called gp55. Proliferation in Friend disease is caused by an interaction between gp55 and a naturally expressed, truncated form of the Stk receptor tyrosine kinase.6 Similar to BCR-ABL–expressing leukemia stem cells, Kit+Sca1+gp55+ Friend leukemia stem cells are sensitive to Δ12-PGJ3–induced apoptosis. Arachodonic acid–derived PGJ2 metabolites also have an antileukemic effect, similar to eicosapentaenoic acid–derived PGJ3 metabolites, although differences may exist in their mechanism of action. Notably, in either case only metabolites with an alkylidene-cyclopentenone structure and unsaturation at carbon-12 are active.
In Friend disease, the uncontrolled proliferation of virus-producing cells causes acute erythroblastosis. Repeated cycles of infection eventually lead to virus integration events, up-regulation of Spi1, and frank erythroleukemia.7 In the acute phase, the treatment of Friend virus–infected mice with Δ12-PGJ3 prevents erythroblastosis in vivo. Further, Δ12-PGJ3 treatment prevents the development of leukemia in Stk-deficient mice transplanted with Friend leukemic stem cells. Thus, Δ12-PGJ3 prevents the development of Friend virus–induced erythroblastosis and erythroleukemia in vivo, and Δ12-PGJ3 exerts a direct effect on leukemic stem cells rather than preventing infection of new cells (because host Stk-deficient cells are intrinsically resistant to the disease). A similar antileukemic effect is observed in mice transplanted with BCR-ABL–transduced cells and subsequently treated with Δ12-PGJ3. In both instances, Δ12-PGJ3 treatment prevents the transfer of leukemia to secondary transplant recipients, indicating that the leukemic stem cell population has been eradicated.
Hegde et al show that leukemic stem cell apoptosis, induced by active metabolites of PGJ2 and PGJ3, correlates with an increase in p53 transcription, phosphorylation, and nuclear translocation. They also show that the ATM target Chk2 is up-regulated and phosphorylated, and that inhibitors of ATM prevent the up-regulation of p53 and apoptosis. Together, these results suggest that PGJ2 and PGJ3 metabolites cause leukemic cell apoptosis by activating the ATM-p53 signaling pathway; however, whether this is the sole mechanism responsible for cell death after treatment with these compounds is not conclusively established. Furthermore, the mechanism whereby Δ12-PGJ3 specifically activates ATM in leukemic cells remains to be determined. Finally, it will be important to extend these encouraging preliminary results to human models of acute leukemia.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal