Abstract
Natural killer (NK) cells are innate immune lymphocytes that provide critical defense against virally infected and transformed cells. NK-cell cytotoxicity requires the formation of an F-actin rich immunologic synapse (IS), as well as the polarization of perforin-containing lytic granules to the IS and secretion of their contents at the IS. It was reported previously that NK-cell cytotoxicity requires nonmuscle myosin IIA function and that granule-associated myosin IIA mediates the interaction of granules with F-actin at the IS. In the present study, we evaluate the nature of the association of myosin IIA with lytic granules. Using NK cells from patients with mutations in myosin IIA, we found that the nonhelical tailpiece is required for NK-cell cytotoxicity and for the phosphorylation of granule-associated myosin IIA. Ultra-resolution imaging techniques demonstrated that single myosin IIA molecules associate with NK-cell lytic granules via the nonhelical tailpiece. Phosphorylation of myosin IIA at residue serine 1943 (S1943) in the tailpiece is needed for this linkage. This defines a novel mechanism for myosin II function, in which myosin IIA can act as a single-molecule actin motor, claiming granules as cargo through tail-dependent phosphorylation for the execution of a pre-final step in human NK-cell cytotoxicity.
Introduction
Natural killer (NK) cells are lymphocytes of the innate immune system that provide critical defense against viral infections and cancer. NK cells respond to infected or transformed cells by directly killing the target cells and also secrete cytokines and provide costimulation to promote immune responses (reviewed in Vivier et al1 ). NK-cell activation takes place in a series of steps, beginning with adhesion signaling and the dynein-mediated convergence of lytic granules to the microtubule organizing center.2-4 After early adhesion signaling, the NK cell forms an array of adhesion and activation receptors called the immunologic synapse (IS; reviewed in Orange5 ). The IS is stabilized by these receptor interactions, as well as the polymerization of a dense layer of filamentous actin (F-actin) at the contact site.6 Once an NK cell forms a mature IS, NK-cell activation directs the polarization of lytic granules and the microtubule organizing center to the IS.3 When the NK-cell secretory machinery has polarized in the direction of a target cell, lytic granule contents, including the pore-forming molecule perforin and apoptosis-inducing granzymes, are secreted at the plasma membrane and direct target cell lysis (reviewed in Hoves et al7 ).
Although NK-cell lytic granules are delivered to the IS via association with microtubules and can dock at the plasma membrane for secretion, the dense layer of F-actin at the IS has the potential to present a barrier to secretion of granule contents at the plasma membrane (reviewed in Sanborn and Orange8 ). Therefore, transport across actin filaments may be required for lytic granules to reach the plasma membrane. As ATP-dependent motor molecules that generate movement across actin filaments, members of the myosin superfamily are ideal for this role. Myosin superfamily members have critical roles in immune cells, and are required for cell motility, migration, and adhesion.9-14 In NK cells, nonmuscle myosin IIA has been defined as a critical regulator of NK-cell cytotoxicity.10,15 Myosin IIA is a hexameric protein composed of 2 heavy chains, 2 regulatory light chains, and 2 essential light chains (reviewed in Eddinger and Meer16 ). The N-terminal head portion of the heavy chain is important for its ATPase function and actin binding, whereas the C-terminal rod domain and nonhelical tailpiece are critical for regulating filament formation and cargo binding (reviewed in Eddinger and Meer16 and Ricketson et al17 ). Myosin IIA function is required for NK-cell cytotoxicity, because NK cells treated with the myosin II inhibitor blebbistatin or with siRNA against myosin IIA have reduced cytotoxicity against target cells.10 Although myosin IIA is not required for conjugation with target cells, IS formation, or lytic granule polarization to the IS,10 it does associate with NK-cell lytic granules.15 The motor function and tailpiece of myosin IIA are required for the interaction of lytic granules with F-actin,15 and therefore the requirement for myosin IIA is specific to lytic granule exocytosis because of its role in enabling granule localization into F-actin at the IS. The mechanism for the requirement for the tailpiece and the nature of the association of myosin IIA with lytic granules, however, remain unclear.
Mutations in the myosin IIA heavy chain (MYH9) cause several disorders, including May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome, which are now collectively known as MYH9-related disease (MYH9-RD). MYH9-RD is characterized by macrothrombocytopenia and leukocyte inclusions, with mutations in some regions of the protein causing the occurrence of additional symptoms such as cataracts, deafness, and glomerulonephritis.18 Whereas immune function is not typically evaluated in patients with MYH9-RD, we previously identified a deficiency in NK-cell cytotoxicity in patients with a C-terminal truncation of MYH9 at position 1933.15 In the present study, we evaluated the role of each major functional region of myosin IIA in NK-cell cytotoxicity using NK cells from patients with MYH9-RD, and define a specific requirement for the myosin IIA head domain and nonhelical tailpiece. We further demonstrated that myosin IIA interacts with lytic granules as a single molecule rather than as a filament, an interaction that is regulated by phosphorylation of the myosin IIA tailpiece at residue serine 1943 (S1943).
Methods
Blood samples and ex vivo NK-cell preparation
Ex vivo NK (eNK) cells were prepared from whole blood obtained from patients or healthy volunteer donors by negative selection using the RosetteSep human NK-cell enrichment cocktail (StemCell Technologies) and centrifugation through Ficoll-Paque Plus lymphocyte isolation medium (Amersham Biosciences) and were used in experiments immediately after preparation without culture in stimulating cytokines or growth factors. For samples from patients with MYH9-related diseases, mutation analyses were performed at the Department of Genetics and Genomic Sciences at Mount Sinai School of Medicine according to published methods.19,20 For biochemical evaluations of eNK-cell lytic granules, cells were prepared as described above from apheresis products obtained from individuals with hemoglobinopathies, which do not substantially alter the NK-cell granule content (data not shown). All human samples were obtained with the approval of the internal institutional review board for the protection of human subjects at the Children's Hospital of Philadelphia.
YTS and target cell lines and cellular evaluation
The immortalized NK cell lines YTS, YTS stably expressing green fluorescent protein (YTS-GFP), or one of 3 different myosin IIA-GFP fusion proteins—full-length (YTS-MyoIIA-GFP), 1933 truncated (YTS-1933x-GFP), or S1943A mutant (YTS-S1943A-GFP)—were used as model NK-cell systems. These were generated using a CMV-GFP-MYH9 plasmid (Addgene) under G418 selection as described previously,15,21 or were generated via those methods using DNA expression constructs containing MYH9-1933x-GFP or MYH9-S1943A-GFP with C5797T (1933x) or T5827G (S1943A) alterations. After establishment of these cell lines, clones of each line that demonstrated equivalent expression of GFP-myosin IIA were selected. GFP expression was monitored by FACS analysis and equivalent expression was maintained by cell sorting. K562 erythroleukemia cells stably expressing CD86 (KT86) and 721.221 B-lymphoblastoid cells were used as target cells.
Flow cytometric, conjugation, and degranulation analyses
eNK cells from patients, healthy volunteer donors, or apheresis donors were analyzed for the expression of CD56 and CD3 using fluorophore-conjugated mAbs (BD Biosciences) and contained a mean purity of 72% CD56+CD3− cells. Where indicated, viability was measured by staining with annexin V-PE and 7-amino-actinomycin D (BD Biosciences). For analysis of NK-cell conjugation with target cells and degranulation, 5 × 105 721.221 target cells were labeled with 50μM PKH26 membrane dye (Sigma-Aldrich) for 5 minutes at 25°C, followed by the addition of FBS. Labeled target cells were then washed and allowed to conjugate with GFP-myosin–expressing YTS cells at a 1:2 ratio in the presence of 1.2 ng/μL of PE-Cy5-conjugated CD107a antibody (eBioscience) at 37°C, after which conjugates were fixed with 1% paraformaldehyde and evaluated by FACS.
51Cr-release cytotoxicity assay
Cytolytic activity of NK-cell lines and eNK cells was measured by 51Cr release, as described previously.19
Isolation of lytic granules from NK cells
Lytic granules were isolated from crude lysosomal fractions of NK cells as described previously.15
Immunoprecipitation
For immunoprecipitation of GFP-tagged myosin from whole-cell lysate, 3 × 106 GFP-myosin–expressing YTS cells were lysed in ice-cold lysis buffer (25mM Tris-Cl, pH 7.5, 150mM NaCl, 5mM MgCl2, 1% Nonidet P-40, 1mM DTT, and 5% glycerol), precleared by centrifugation at 14 000g, and then incubated at 4°C for 1 hour with Protein G-Agarose beads (Invitrogen) and anti-GFP antibody (Clontech 632460). For immunoprecipitation from lytic granules, purified granules were resuspended in 2M NaCl for 20 minutes at 25°C, subjected to centrifugation at 18 000g, and supernatants used for immunoprecipitation as above.
Western blot analyses
Western blot analysis of total lysate, isolated lytic granules, and immunoprecipitates, as well as detection of bound antibodies, were performed as described previously,15 except that the membrane was incubated with anti-myosin IIA (Sigma-Aldrich M8064, C-terminal epitope), anti-myosin IIA (Abcam 55 456, internal epitope), anti-myosin IIA phosphoserine 1943 (ECM Biosciences), anti-GFP (Clontech), or anti-granzyme B (Sigma-Aldrich) antibodies.
NK cell IFN-γ secretion
YTS NK cells were added to medium or 721.221 target cells at a 2:1 effector to target cell ratio for 0 or 24 hours. After incubation, cell supernatants were frozen at −80°C and batch analyzed by IFN-γ ELISA (R&D Systems).
Fixed-cell microscopy
Samples were prepared and fixed-cell imaging performed using a spinning-disk confocal microscope (Olympus IX-81 DSU), as described previously.15 Alexa Fluor 568 phalloidin (Invitrogen) and mouse anti–perforin antibody (clone δG9; BD Biosciences), followed by Pacific Blue conjugated goat anti–mouse antibody (Invitrogen), were used for visualization of F-actin and perforin, respectively. Images were analyzed using Volocity Version 5.5 software (Improvision). To calculate accumulated fluorophores, thresholds for GFP-myosin signal were set as 2 standard deviations above the mean fluorescence intensity of the field, and for perforin using a fixed value based on isotype controls. Colocalization was determined in > 20 images per cell type by comparing the volume of perforin and GFP co-occupancy with the total volume of perforin signal.
Electron microscopy
Electron microscopy of isolated lytic granules was performed by platinum rotary shadowing as described previously,22 except that the sample consisted of purified lytic granules from YTS cells that were adhered to glass coverslips coated with anti-myosin IIA antibody (Sigma-Aldrich).
STED microscopy
YTS cell lytic granules were adhered to poly-L-lysine–coated glass slides and prepared as for confocal microscopy as described previously,15 except slides were treated with rabbit anti–myosin IIA antibody (Sigma-Aldrich) followed by DyLight 488–conjugated goat anti–rabbit antibody (BioLegend). For fixed-cell stimulated emission depletion (STED) microscopy, samples were prepared as described previously for confocal microscopy (see above) except using anti-perforin AlexaFluor 647 (BioLegend) and rabbit anti–myosin primary antibody, followed by goat anti–rabbit DyLight 488 secondary antibody. STED images were acquired with a Leica STED CW system using an HCX APO 100×/1.4 oil STED objective. All samples were mounted in Prolong anti-fade mounting medium (Invitrogen). DyLight 488 was excited using a 488-nm argon laser, and STED depletion was achieved using a 592-nm continuous-wave fiber laser. AlexaFlour 647 was excited using an HeNe 633 laser and imaged using the laser scanning confocal modality of the system. For fixed-cell imaging, fluorescence was detected with HyD detectors (Leica). Image analysis was performed using LAS AF Lite Version 1.9 (Leica) and ImageJ Version 1.44 (National Institutes of Health) software.
Statistics
For fixed-cell microscopy, the minimum number of cells evaluated was determined using a sample size calculation based on preliminary data with α and β error levels of 1%. Differences between cell types were determined using an unpaired 2-tailed Student t test or Wilcoxon matched pairs test. Differences were considered significant if P < .05.
Results
Human NK cells with mutations in the myosin IIA head or tailpiece have impaired cytotoxicity
We have demonstrated previously that a truncation mutation in the tailpiece of myosin IIA reduces NK-cell cytotoxic function.15 To further examine the role of each region of myosin IIA in NK-cell function, we evaluated patients with MYH9-RD due to mutations in each portion of MYH9. Common clinical findings in MYH9-RD include macrothrombocytopenia, leukocyte inclusions, cataracts, deafness, and nephritis.18 The patients in this study demonstrated some or all of these symptoms (Table 1), in accordance with previous reports of variable effects of MYH9 mutations.23-25 To determine the effect of these mutations on NK-cell cytotoxicity, eNK cells from these patients were prepared from peripheral blood and immediately evaluated for their ability to kill K562 erythroleukemia cells. Cytotoxicity of the NK-cell preparations was adjusted for purity of the patient and control NK-cell preparations, and the cytotoxic activity of each patient's NK cells was compared with the shipping control for the patient using lytic unit calculations to control for variability in shipping and assay conditions.
Mutations and phenotypes of patients with MYH9-RD
| Patient no. . | Mutation . | Macro-thrombocytopenia . | Döhle bodies . | Hearing loss . | Nephritis . | Cataracts . |
|---|---|---|---|---|---|---|
| P1 | S96L | Y | N | N | Y | N |
| P2 | T1155I | Y | Y | Y | Y | Y |
| P3 | R1400W | Y | ND | N | N | N |
| P4 | D1424N | Y | ND | N | N | N |
| P5 | D1424N | Y | ND | Y | N | N |
| P6 | D1424N | Y | Y | N | N | N |
| P7 | 5779delC | Y | ND | N | N | N |
| P8 | E1945x | Y | Y | N | N | N |
| Patient no. . | Mutation . | Macro-thrombocytopenia . | Döhle bodies . | Hearing loss . | Nephritis . | Cataracts . |
|---|---|---|---|---|---|---|
| P1 | S96L | Y | N | N | Y | N |
| P2 | T1155I | Y | Y | Y | Y | Y |
| P3 | R1400W | Y | ND | N | N | N |
| P4 | D1424N | Y | ND | N | N | N |
| P5 | D1424N | Y | ND | Y | N | N |
| P6 | D1424N | Y | Y | N | N | N |
| P7 | 5779delC | Y | ND | N | N | N |
| P8 | E1945x | Y | Y | N | N | N |
The position of the mutation for each patient with MYH9-RD is listed, along with the symptoms present or absent.
ND indicates not determined.
The N-terminal head region of MYH9 is critical for binding to F-actin and ATP hydrolysis for motor function, which requires the flexibility of the neighboring subfragment 2 (S2) portion for myosin head motility.16 NK cells derived from patients with the S96L mutation in the head region and T1155I mutation in the S2 region (Figure 1A) demonstrated a reduced ability to lyse K562 target cells compared with control eNK cells (Figure 1B patients P1 and P2), suggesting a role for myosin IIA motor function in NK-cell cytotoxicity, in accordance with previous studies.10,15 In contrast, although the helical rod portion of myosin IIA is critical for formation of bipolar myosin II filaments,17,26 2 mutations in the rod region, R1400W and D1424N (Figure 1A), affected cytotoxicity to a lesser extent (Figure 1B patients P3-P6).
Effect of naturally occurring mutations in MYH9 on human NK-cell cytotoxicity. (A) Schematic depicting the major functional regions of a myosin IIA molecule, indicating the position of MYH9 mutations in patients whose NK cells were used in this study. (B) Cytotoxic activity of negatively selected, purified ex vivo human NK cells from patients with mutations in the head (S96L, patient 1 [P1]), S2 (T1155I, P2), rod (R1400W, P3; D1424N, P4-P6), and tail (5779delC, P7) domains of MYH9 was compared with control donors in 51Cr-release assays against K562 target cells. Results from 1933x patients were published previously15 and are shown for comparison. Results are listed as lytic units required for 10% lysis of target cells (LU10). Results are adjusted for NK-cell purity of the isolated cells, and patient cell LU10 is normalized to control for each assay. For 1933x, when > 1 patient was analyzed, the mean ± SD is shown.
Effect of naturally occurring mutations in MYH9 on human NK-cell cytotoxicity. (A) Schematic depicting the major functional regions of a myosin IIA molecule, indicating the position of MYH9 mutations in patients whose NK cells were used in this study. (B) Cytotoxic activity of negatively selected, purified ex vivo human NK cells from patients with mutations in the head (S96L, patient 1 [P1]), S2 (T1155I, P2), rod (R1400W, P3; D1424N, P4-P6), and tail (5779delC, P7) domains of MYH9 was compared with control donors in 51Cr-release assays against K562 target cells. Results from 1933x patients were published previously15 and are shown for comparison. Results are listed as lytic units required for 10% lysis of target cells (LU10). Results are adjusted for NK-cell purity of the isolated cells, and patient cell LU10 is normalized to control for each assay. For 1933x, when > 1 patient was analyzed, the mean ± SD is shown.
The nonhelical tailpiece at the C-terminus of myosin IIA is required for binding to cargo, as well as for regulation of the protein.27-30 This area can also bind to regulatory elements at residues 1916 and 1943, as well as to phospholipid membranes.11,28,31-33 We demonstrated previously that a truncation of the nonhelical tailpiece at residue 1933 diminishes NK-cell cytotoxicity.15 Like cells from patients with the 1933x truncation mutation, eNK cells from a patient with the 5779delC mutation (Figure 1A), which causes a frameshift at residue 1927 that results in a truncation of the protein at residue 1942,34 have reduced cytotoxic function compared with control cells (Figure 1B patient P7). These results point to a specific requirement for myosin IIA motor function and its nonhelical tailpiece in NK-cell cytotoxicity.
The myosin IIA tailpiece is phosphorylated at S1943
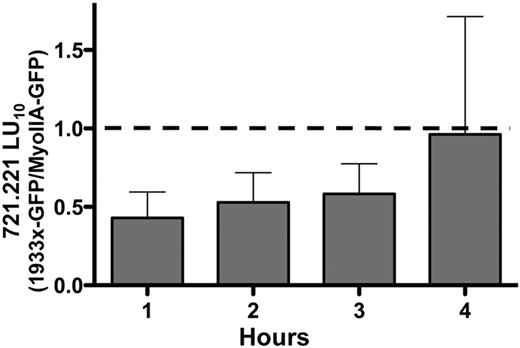
Results from patients with MYH9-RD suggested a specific requirement for the MYH9 tailpiece in NK-cell cytotoxicity. Therefore, to determine the nature of this requirement, we generated an NK-cell model system with which to study the role of the MYH9 tailpiece and created a YTS NK cell line expressing the MYH9 1933x truncation (hereafter referred to as 1933x) as a GFP fusion protein (YTS-1933x-GFP). These cells expressed the 1933x-GFP protein at levels of approximately 20% that of endogenous myosin IIA (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Similar to patients with the 1933x mutation, the presence of the mutant myosin in YTS-1933x-GFP cells led to reduced cytotoxicity compared with cells expressing a wild-type myosin GFP fusion protein (YTS-MyoIIA-GFP). This was most readily apparent in 51Cr-release assays against 721.221 target cells with incubation times < 4 hours (Figure 2 and supplemental Figure 2). This may be due to the presence of a smaller ratio of 1933x myosin to endogenous, nonmutated myosin IIA in the cell line (20% of endogenous), in contrast to cells from patients, which are heterozygous for the 1933x mutation.
The 1933x mutation in MYH9 reduces NK-cell killing. Cytotoxic activity of the YTS NK cell line stably expressing a 1933x myosin IIA-GFP fusion protein was compared with YTS cells expressing wild-type myosin IIA-GFP in 51Cr-release assays against 721.221 target cells. Results are listed as lytic units required for 10% lysis of target cells (LU10), and 1933x-GFP LU10 is normalized to myosin IIA-GFP for each assay. Results shown are the mean of 3 experiments and error bars indicate SD.
The 1933x mutation in MYH9 reduces NK-cell killing. Cytotoxic activity of the YTS NK cell line stably expressing a 1933x myosin IIA-GFP fusion protein was compared with YTS cells expressing wild-type myosin IIA-GFP in 51Cr-release assays against 721.221 target cells. Results are listed as lytic units required for 10% lysis of target cells (LU10), and 1933x-GFP LU10 is normalized to myosin IIA-GFP for each assay. Results shown are the mean of 3 experiments and error bars indicate SD.
The tailpiece of myosin IIA is a site of regulation by phosphorylation in many cell types.9,11,35,36 To evaluate the phosphorylation of the myosin tailpiece, we immunoprecipitated the GFP-tagged wild-type and 1933x myosins from resting YTS cells and YTS cells conjugated to KT86 target cells and compared their phosphorylation states. In all cases, GFP immunoprecipitation resulted in the isolation of both GFP-tagged and untagged myosin IIA (Figure 3A upper band is the GFP fusion protein and lower band is endogenous), indicating that the 2 populations of myosin in the cells were able to complex with each other. In GFP immunoprecipitates from cells expressing wild-type myosin IIA, gel staining for phosphorylation revealed substantial phosphorylation of the GFP-tagged myosin IIA and the endogenous protein (Figure 3A). The amount of phosphorylation did not change upon conjugation with target cells. Compared with YTS-MyoIIA-GFP cells, YTS-1933x-GFP cells demonstrated reduced phosphorylation of the GFP-tagged protein, although equivalent quantities of protein were precipitated and the endogenous protein remained phosphorylated (Figure 3A). Because we had previously defined an association of myosin IIA with NK-cell lytic granules,15 we next wanted to specifically evaluate lytic granule–associated myosin IIA. Therefore, granules were isolated from YTS-MyoIIA-GFP and YTS-1933x-GFP cells and treated with 2M NaCl to remove peripherally associated proteins for immunoprecipitation. Similar to whole cells, immunoprecipitation of GFP-tagged myosins from lytic granule–associated proteins demonstrated that granule-associated myosin IIA is constitutively phosphorylated and that the 1933x mutation decreases phosphorylation (Figure 3B). This suggests that the MYH9 tailpiece is a site of myosin IIA phosphorylation in NK cells.
Myosin IIA is constitutively phosphorylated at S1943. (A-D) YTS cells expressing wild-type myosin IIA-GFP (MIIA) or 1933x myosin IIA-GFP (1933x) were left resting or were conjugated to KT86 target cells. GFP-tagged proteins were immunoprecipitated from lysates (A,C) or from the supernatants of isolated lytic granules that were treated with NaCl to remove surface proteins (B,D). GFP immunoprecipitates were evaluated by gel staining for phosphorylation (Pro-Q) and total protein (Sypro) (A-B), or by Western blot for phospho-S1943 and total myosin IIA (C-D). In all gels of GFP immunoprecipitates, lanes were loaded for equal GFP-myosin content to facilitate comparison of phosphorylation, and therefore the 1933x lanes contain greater total protein. In panels A through D, numbers below blots indicate the ratio of the phosphorylated band intensity to the total protein intensity for the GFP-tagged protein (upper band) normalized to the ratio for myosin IIA. In each image, there are 2 myosin bands: the larger corresponds to 253 kDa and represents the myosin IIA GFP fusion, which is enriched in the immunoprecipitates; the smaller corresponds to 227 kDa and represents the endogenous myosin IIA. The inclusion of 2 heavy chains in each myosin hexamer enables association of exogenous and endogenously expressed myosin IIA molecules when evaluated via immunoprecipitation (see also supplemental Figure 1). (E) Resting and stimulated ex vivo human NK-cell lysates and lytic granules isolated from resting eNK cells evaluated by Western blot for phospho-S1943 and total myosin IIA. Results are representative of 3 (A-E, eNK) or 2 (E, LG) independent assays.
Myosin IIA is constitutively phosphorylated at S1943. (A-D) YTS cells expressing wild-type myosin IIA-GFP (MIIA) or 1933x myosin IIA-GFP (1933x) were left resting or were conjugated to KT86 target cells. GFP-tagged proteins were immunoprecipitated from lysates (A,C) or from the supernatants of isolated lytic granules that were treated with NaCl to remove surface proteins (B,D). GFP immunoprecipitates were evaluated by gel staining for phosphorylation (Pro-Q) and total protein (Sypro) (A-B), or by Western blot for phospho-S1943 and total myosin IIA (C-D). In all gels of GFP immunoprecipitates, lanes were loaded for equal GFP-myosin content to facilitate comparison of phosphorylation, and therefore the 1933x lanes contain greater total protein. In panels A through D, numbers below blots indicate the ratio of the phosphorylated band intensity to the total protein intensity for the GFP-tagged protein (upper band) normalized to the ratio for myosin IIA. In each image, there are 2 myosin bands: the larger corresponds to 253 kDa and represents the myosin IIA GFP fusion, which is enriched in the immunoprecipitates; the smaller corresponds to 227 kDa and represents the endogenous myosin IIA. The inclusion of 2 heavy chains in each myosin hexamer enables association of exogenous and endogenously expressed myosin IIA molecules when evaluated via immunoprecipitation (see also supplemental Figure 1). (E) Resting and stimulated ex vivo human NK-cell lysates and lytic granules isolated from resting eNK cells evaluated by Western blot for phospho-S1943 and total myosin IIA. Results are representative of 3 (A-E, eNK) or 2 (E, LG) independent assays.
Because residue S1943 is a key site of myosin IIA phosphorylation28 and is removed by the 1933x truncation, we wanted to determine whether S1943 is phosphorylated in NK cells. Using GFP immunoprecipitates from whole-cell lysate and isolated lytic granules, S1943 phosphorylation was evaluated in MyoIIA-GFP- and 1933x-GFP–expressing YTS cells with an antibody specific for phospho-S1943. Wild-type myosin IIA was constitutively phosphorylated at S1943 in both whole-cell lysate and isolated lytic granules (Figure 3C-D). Because of the truncation upstream of the residue in 1933x myosin, the 1933x-GFP protein did not contain S1943 and thus did not demonstrate S1943 phosphorylation. The presence of the mutated myosin did not, however, affect phosphorylation of S1943 in endogenous myosin IIA (Figure 3C-D). To ensure that this phosphorylation pattern was not specific to our cell line and was common to human NK cells, phosphorylation of myosin IIA was evaluated in human eNK cells and in their lytic granules. In both, myosin IIA was also phosphorylated at S1943 (Figure 3E), suggesting that the regulation of myosin observed in YTS cell lines is similar to that in primary human NK cells and that the MYH9 tailpiece was required for its phosphorylation.
Phosphorylation of myosin IIA at S1943 is required for NK-cell cytotoxicity
Because S1943 is phosphorylated in NK cells, and cells expressing truncated myosin IIA and lacking this residue (1933x) were impaired in cytotoxic activity, we wanted to determine whether there was a specific requirement for S1943 phosphorylation in NK-cell function. Therefore, we generated a YTS cell line expressing GFP-tagged myosin IIA with an S1943A mutation, which would therefore be unphosphorylatable at residue 1943 (YTS-S1943A-GFP).
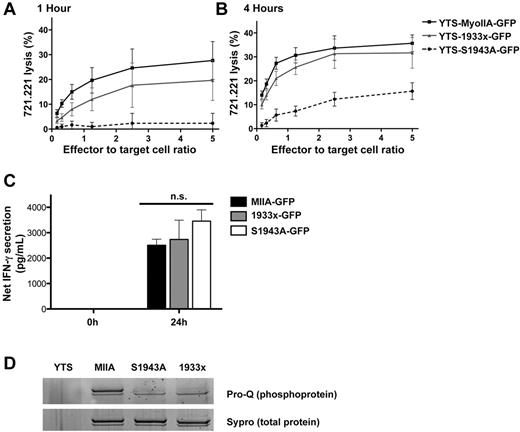
These cells expressed GFP-tagged S1943A myosin IIA at ∼ 20% of endogenous myosin levels, similar to MyoIIA-GFP and 1933x-GFP cells (supplemental Figure 1). The YTS-S1943A-GFP cells, however, had reduced levels of cytotoxicity compared with both MyoIIA-GFP and 1933x-GFP cells (Figure 4A-B). This reduction in cytotoxic activity of S1943A-GFP cells was present in both the 1- and the 4-hour assays, although some activity was detectable at 4 hours. The reduced killing in these cells was not due to impaired viability, because all cell lines were > 95% viable by flow cytometry for annexin V and 7-amino-actinomycin D (not shown). In addition, the YTS-S1943A-GFP cells were as capable of conjugating to 721.221 target cells as MyoIIA-GFP and 1933x-GFP cells (supplemental Figure 3) and also secreted IFN-γ at similar levels upon conjugation (Figure 4C), suggesting that other effector functions requiring NK-cell activation were intact. Compared with YTS-MyoIIA-GFP cells, S1943A-GFP cells demonstrated reduced phosphorylation of the GFP-tagged protein in GFP immunoprecipitates (Figure 4D). This effect was similar to that in the 1933x-GFP–expressing cells. These results suggest that S1943 is a primary site for phosphorylation in myosin IIA, which is specifically required for NK-cell cytotoxicity.
Myosin IIA phosphorylation at S1943 is required for NK-cell cytotoxicity, but not IFN-γ secretion. Cytotoxic activity (A-B) and IFN-γ secretion (C) of YTS cells expressing wild-type myosin IIA-GFP (YTS-MyoIIA-GFP), 1933x myosin IIA-GFP (YTS-1933x-GFP), or S1943A myosin IIA-GFP (YTS-S1943A-GFP) was measured against 721.221 target cells. Cytotoxicity was measured at 1 hour (A) and 4 hours (B) after conjugation by 51Cr-release assay. (C) IFN-γ secretion into the supernatant was measured 24 hours after conjugation by ELISA and calculated by subtracting IFN-γ secretion of NK cells alone. In all experiments, results are the mean of 3 independent assays and error bars indicate SD. (D) GFP-tagged proteins were immunoprecipitated from lysates from YTS cells or YTS cells expressing wild-type myosin IIA-GFP (MIIA), S1943A myosin IIA-GFP (S1943A), or 1933x myosin IIA-GFP (1933x). GFP immunoprecipitates were evaluated by gel staining for total phosphorylation (Pro-Q) and total protein (Sypro). The larger of the 2 bands represents the myosin IIA-GFP fusion, which is enriched in the immunoprecipitates, and the smaller the endogenous myosin IIA. Results are representative of 3 independent experiments.
Myosin IIA phosphorylation at S1943 is required for NK-cell cytotoxicity, but not IFN-γ secretion. Cytotoxic activity (A-B) and IFN-γ secretion (C) of YTS cells expressing wild-type myosin IIA-GFP (YTS-MyoIIA-GFP), 1933x myosin IIA-GFP (YTS-1933x-GFP), or S1943A myosin IIA-GFP (YTS-S1943A-GFP) was measured against 721.221 target cells. Cytotoxicity was measured at 1 hour (A) and 4 hours (B) after conjugation by 51Cr-release assay. (C) IFN-γ secretion into the supernatant was measured 24 hours after conjugation by ELISA and calculated by subtracting IFN-γ secretion of NK cells alone. In all experiments, results are the mean of 3 independent assays and error bars indicate SD. (D) GFP-tagged proteins were immunoprecipitated from lysates from YTS cells or YTS cells expressing wild-type myosin IIA-GFP (MIIA), S1943A myosin IIA-GFP (S1943A), or 1933x myosin IIA-GFP (1933x). GFP immunoprecipitates were evaluated by gel staining for total phosphorylation (Pro-Q) and total protein (Sypro). The larger of the 2 bands represents the myosin IIA-GFP fusion, which is enriched in the immunoprecipitates, and the smaller the endogenous myosin IIA. Results are representative of 3 independent experiments.
S1943 phosphorylation mediates the interaction of myosin IIA with lytic granules
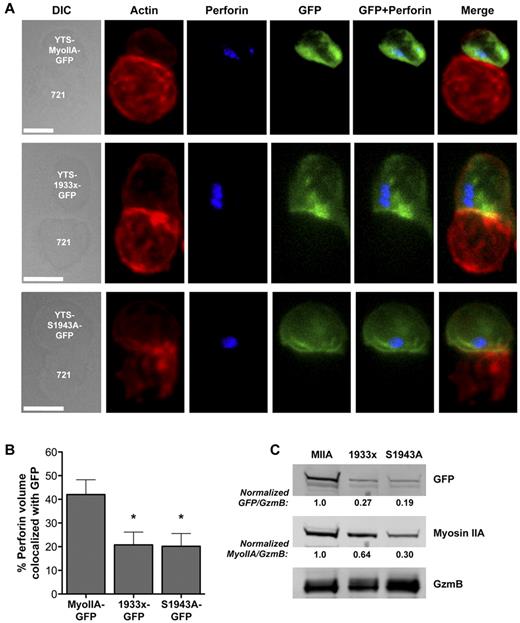
To determine the mechanism responsible for the impact of S1943A-GFP myosin expression on NK-cell killing, we examined conjugates of MyoIIA-GFP–, 1933x-GFP–, and S1943A-GFP–expressing YTS cells with 721.221 target cells using confocal immunofluorescence microscopy. Compared with the YTS-MyoIIA-GFP cells, 1933x-GFP and S1943A-GFP cells formed equally mature synapses with target cells, with polymerization of F-actin at the IS and polarization of GFP-tagged myosin to the region of F-actin accumulation (Figure 5A). Whereas perforin also polarized to the IS in cells expressing the mutant myosins, colocalization of perforin fluorescence with GFP fluorescence was diminished in 1933x-GFP and S1943A-GFP cells compared with YTS-MyoIIA-GFP (Figure 5B, 42.05% perforin volume colocalized with GFP in MyoIIA-GFP vs 20.77% and 20.15% in 1933x- and S1943A-GFP, respectively). This suggests that the mutant myosin molecules were less capable of associating with NK-cell lytic granules. The ability of the GFP-tagged myosin mutants to associate with lytic granules was evaluated using Western blot analysis of isolated lytic granules from YTS-MyoIIA-GFP, 1933x-GFP, and S1943A-GFP cells. Compared with YTS-MyoIIA-GFP cells, the GFP signal as a feature of total granule protein was reduced in granules from 1933x-GFP and S1943A-GFP cells (Figure 5C GFP/GzmB ratio), indicating that the association of the myosin mutants with lytic granules was reduced. The total amount of detectable myosin IIA was reduced substantially in the 1933x and to a greater extent in the S1943A lytic granules (Figure 5C MyoIIA/GzmB ratio), suggesting that the mutant myosins may prevent the association of endogenous myosin IIA with lytic granules. To specifically evaluate the role of S1943 phosphorylation in the clinical setting, we evaluated NK-cell cytotoxicity in patients with a truncation mutation removing the tailpiece downstream of S1943 (E1945x, patient P8). Although we were unable to obtain enough cells to evaluate isolated NK cells from this patient, the patient demonstrated normal PBMC cytotoxicity compared with control (supplemental Figure 4), indicating that the portion of the tailpiece downstream of S1943 was less required for cytotoxicity. Overall, these data suggest that an intact myosin IIA tailpiece and phosphorylation of myosin IIA are required for association with lytic granules.
The myosin IIA tailpiece and S1943 phosphorylation are required for its interaction with lytic granules. (A) Confocal fluorescent micrographs of YTS cells expressing wild-type myosin IIA-GFP (MyoIIA-GFP), 1933x myosin IIA-GFP (1933x-GFP), or S1943A myosin IIA-GFP (S1943A-GFP) conjugated to 721.221 target cells (721) Differential interference contrast (DIC, left) in addition to fluorescent signal for actin (red), perforin (blue), or GFP-myosin (green) are shown, along with an overlay of fluorescent channels (right). Scale bar indicates 5 μm. (B) Percentage of total perforin fluorescent volume colocalized with GFP volume ± SD; > 20 cells per condition were analyzed. (C) Western blot of isolated lytic granules from resting YTS cells expressing wild-type myosin IIA-GFP (MIIA), 1933x myosin IIA-GFP (1933x), or S1943A myosin IIA-GFP (S1943A). GFP, myosin IIA, and granzyme B (GzmB) blots are shown. Gel was loaded for equal granule protein content to facilitate comparison of GFP-myosin quantity on lytic granules. Numbers below blots indicate the ratio of the GFP, and the myosin band intensities to the total intensity for granzyme B normalized to the ratio for MIIA. Results shown are representative of 3 independent assays.
The myosin IIA tailpiece and S1943 phosphorylation are required for its interaction with lytic granules. (A) Confocal fluorescent micrographs of YTS cells expressing wild-type myosin IIA-GFP (MyoIIA-GFP), 1933x myosin IIA-GFP (1933x-GFP), or S1943A myosin IIA-GFP (S1943A-GFP) conjugated to 721.221 target cells (721) Differential interference contrast (DIC, left) in addition to fluorescent signal for actin (red), perforin (blue), or GFP-myosin (green) are shown, along with an overlay of fluorescent channels (right). Scale bar indicates 5 μm. (B) Percentage of total perforin fluorescent volume colocalized with GFP volume ± SD; > 20 cells per condition were analyzed. (C) Western blot of isolated lytic granules from resting YTS cells expressing wild-type myosin IIA-GFP (MIIA), 1933x myosin IIA-GFP (1933x), or S1943A myosin IIA-GFP (S1943A). GFP, myosin IIA, and granzyme B (GzmB) blots are shown. Gel was loaded for equal granule protein content to facilitate comparison of GFP-myosin quantity on lytic granules. Numbers below blots indicate the ratio of the GFP, and the myosin band intensities to the total intensity for granzyme B normalized to the ratio for MIIA. Results shown are representative of 3 independent assays.
Myosin IIA associates with lytic granules in single-molecule form via the tailpiece
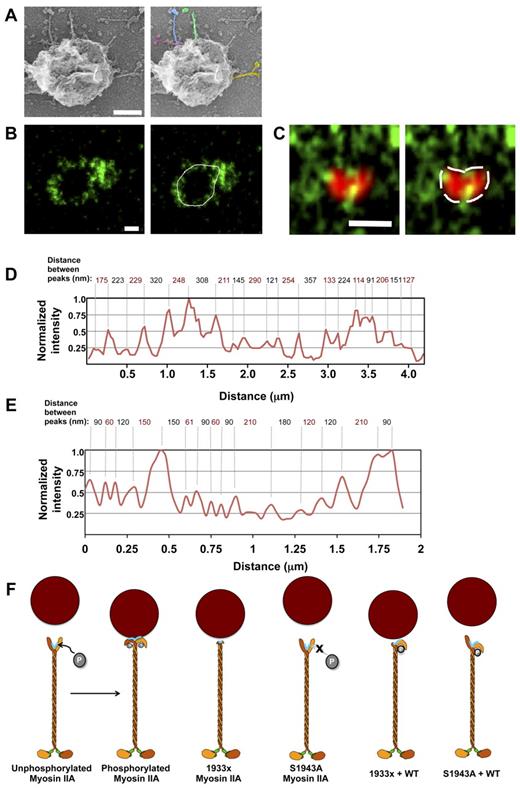
Because phosphorylation of myosin IIA via its tailpiece was required for its linkage to lytic granules, we sought to determine whether myosin IIA associates with granules via the tailpiece directly. Although many myosin classes are known to associate with cargo in single-molecule form using the tail for cargo binding,37 myosin II family members are typically considered to exist in filaments. To determine the mechanism of myosin IIA association with lytic granules, granules were isolated from YTS NK cells and prepared for platinum rotary shadowing electron microscopy, a technique that highlights 3D structures.38 The lytic granules, which were adhered to glass coated with anti-myosin IIA antibody, demonstrated emanating structures with an appearance and dimensions characteristic of myosin IIA molecules (Figure 6A). The lytic granules measured an average of 420 nm in diameter, which is consistent with estimates of lytic granule size. Similarly, the long, rod-like domains of the associated structures had a mean length of 194 nm, which is consistent with the length of the myosin IIA rod domain.33 To verify that the molecules present around the periphery of the lytic granules were myosin IIA and to gain greater insight into their arrangement, isolated granules were evaluated in ultra-resolution using STED microscopy.39 These images had a resolution of 54 nm, as determined by measuring single fluorophore-conjugated antibody signal (not shown). If myosin IIA associated with NK-cell lytic granules in large filamentous assemblies, the distance between tailpiece regions of adjacent molecules would be expected to be a consistent 14.3 nm, equivalent to the stagger of myosin IIA molecules in filaments,40 which would appear as a continuous string of myosin IIA signal surrounding the granule at a 54-nm resolution. In contrast to this prediction, isolated lytic granules labeled with an antibody against the myosin IIA tailpiece demonstrated a distance of approximately 73-380 nm between myosin molecules (Figure 6B,D), with a mean of 179.8 nm over 3 separate analyses. Furthermore, the distance between molecules was irregular (SD of 86 nm in distance between molecules), making any large ordered complex of myosin IIA molecules highly unlikely. To ensure that this large spacing between molecules was not due to the removal of myosin IIA molecules during the lytic granule isolation procedure, lytic granules were also examined within cells. The spacing between molecules around lytic granules was similar within intact cells (Figure 6C,E). These results suggest an association of single irregularly spaced molecules or minifilaments of myosin IIA with lytic granules via the tailpiece, and more broadly define a mechanism by which a prefinal step for human NK-cell cytotoxicity is governed through the tail-dependent phosphorylation and function of lytic granule-associated myosin IIA, which claims granules as cargo.
Single molecules of myosin IIA associate with NK-cell lytic granules. Lytic granules isolated from resting YTS cells were evaluated by platinum rotary shadowing electron microscopy (A). Whole resting YTS cells or lytic granules isolated from resting YTS cells were evaluated by STED microscopy (B-E). (A) YTS cell lytic granule with emanating structures resembling myosin IIA, which are highlighted in color in the image at right. Scale bar indicates 200 nm. (B) YTS cell isolated lytic granule visualized with an antibody against the myosin IIA tailpiece (green, left). (C) Lytic granule within fixed YTS cell visualized with anti-perforin (red, confocal) and anti-myosin (green, STED) antibodies. The distance between myosin IIA antibody molecules was measured using a line drawn around the periphery of the lytic granules (B-C, white, right). Scale bar indicates 500 nm. (D-E) Normalized intensity plots along the line drawn at the periphery of the lytic granules shown in panels B-C. Values are normalized such that the maximum intensity in the plot is equal to 1. Numbers at the top indicate the distance between adjacent antibody molecules. Results are representative of > 10 (A), 3 (B,D), or > 30 (C,E) images. (F) Proposed model of myosin IIA interaction with NK-cell lytic granules. Unphosphorylated myosin IIA tailpiece obscures the binding site for lytic granules (highlighted in turquoise). Myosin IIA tailpiece phosphorylated at S1943 bends backward onto the rod, revealing the putative granule binding site. 1933x myosin IIA is missing a large portion of the granule-binding site, and therefore cannot sustain an interaction with granules under force. S1943A myosin IIA has an intact tailpiece, but cannot be phosphorylated and therefore cannot reveal its putative granule-binding site. Myosin molecules containing one 1933x and one WT myosin are able to bind to granules with a weaker interaction, whereas molecules containing 1 S1943A and 1 wild-type myosin are sterically hindered from binding.
Single molecules of myosin IIA associate with NK-cell lytic granules. Lytic granules isolated from resting YTS cells were evaluated by platinum rotary shadowing electron microscopy (A). Whole resting YTS cells or lytic granules isolated from resting YTS cells were evaluated by STED microscopy (B-E). (A) YTS cell lytic granule with emanating structures resembling myosin IIA, which are highlighted in color in the image at right. Scale bar indicates 200 nm. (B) YTS cell isolated lytic granule visualized with an antibody against the myosin IIA tailpiece (green, left). (C) Lytic granule within fixed YTS cell visualized with anti-perforin (red, confocal) and anti-myosin (green, STED) antibodies. The distance between myosin IIA antibody molecules was measured using a line drawn around the periphery of the lytic granules (B-C, white, right). Scale bar indicates 500 nm. (D-E) Normalized intensity plots along the line drawn at the periphery of the lytic granules shown in panels B-C. Values are normalized such that the maximum intensity in the plot is equal to 1. Numbers at the top indicate the distance between adjacent antibody molecules. Results are representative of > 10 (A), 3 (B,D), or > 30 (C,E) images. (F) Proposed model of myosin IIA interaction with NK-cell lytic granules. Unphosphorylated myosin IIA tailpiece obscures the binding site for lytic granules (highlighted in turquoise). Myosin IIA tailpiece phosphorylated at S1943 bends backward onto the rod, revealing the putative granule binding site. 1933x myosin IIA is missing a large portion of the granule-binding site, and therefore cannot sustain an interaction with granules under force. S1943A myosin IIA has an intact tailpiece, but cannot be phosphorylated and therefore cannot reveal its putative granule-binding site. Myosin molecules containing one 1933x and one WT myosin are able to bind to granules with a weaker interaction, whereas molecules containing 1 S1943A and 1 wild-type myosin are sterically hindered from binding.
Discussion
We demonstrated previously that myosin IIA is associated with NK-cell lytic granules and facilitates the interaction of granules with F-actin at the IS for exocytosis.15 This role for myosin IIA required its motor function and tailpiece, yet the precise mechanism for the association of myosin with the granules and its function there were unclear. In the present study, we sought to further examine the role for myosin IIA in NK-cell lytic granule exocytosis by determining which portions of the protein are required for NK-cell cytotoxicity. Studies of NK cells from patients with different mutations in MYH9 point to a requirement for the motor function and tailpiece, because rod mutations in MYH9 had a lesser effect on NK-cell cytotoxicity (Figure 1 patients P3-P6). Because the rod domain is important for regulating intermolecular interactions during filament formation,17 and because at least one of the mutations examined has been shown to alter the periodicity of myosin IIA rods (D1424N),26 these results suggest that the ability to form bipolar filaments is not required for this function of myosin IIA. These data add potential clinical relevance to the role of myosin IIA in NK-cell killing and raise an additional consideration in patients with MYH9-RD.
Whereas the requirement for the motor domain of myosin IIA in lytic granule exocytosis was likely because of the need to translocate granules across actin filaments,15 the role of the nonhelical tailpiece was less clear. Unlike the 1933x truncation mutation, which removes the entire tailpiece of myosin IIA, a greater portion of the tailpiece remains intact in the 5779delC myosin, which also affected NK-cell killing (Figure 1B patient P7). This mutation also leads to a truncation41 and suggests that the requirement for the tailpiece may be a feature of the amino acid sequence rather than being related to tailpiece or filament structure. The C-terminus of myosin IIA is a key site of phosphorylation in many cell types, including mast cells11 and human T cells.9 To understand the importance of regulation via the myosin IIA tailpiece, we created a YTS NK cell line expressing GFP-tagged 1933x myosin, which demonstrated a cytotoxicity defect only at early time points (Figure 2 and supplemental Figure 2). This may be because of the presence of sufficient endogenous myosin IIA; however, it is also possible that the 1933x myosin is capable of associating weakly with the lytic granules, allowing it to partially assist in granule exocytosis, as discussed below. Mutant myosin from YTS-1933x-GFP cells or lytic granules prepared from these cells demonstrated reduced phosphorylation (Figure 3), suggesting that the tailpiece of myosin IIA is required for phosphorylation of the entire protein. This is because of the phosphorylation of S1943, because this site was phosphorylated in wild-type myosin IIA and was absent in the truncated 1933x protein. S1943 was also phosphorylated in ex vivo human NK cells (Figure 3E), indicating that this mechanism of regulation is not exclusive to our cell line. Furthermore, S1943A myosin-expressing cells formed conjugates with target cells and secreted IFN-γ adequately, but prevented myosin IIA phosphorylation and had diminished cytotoxicity (Figure 4), suggesting that S1943 phosphorylation specifically regulates the function of myosin IIA in lytic granule exocytosis.
Because mutations of the rod domain of myosin IIA, which regulates filament formation, did not affect NK-cell cytotoxicity (Figure 1), the requirement for the tailpiece suggested that it may directly mediate granule attachment using a S1943 phosphorylation–dependent mechanism. This is based on the observation that cells expressing S1943A-GFP myosin demonstrated reduced cytotoxicity and association of the GFP-tagged myosin with perforin-containing granules (Figures 4 and 5). Two different ultra-resolution techniques confirmed that myosin IIA is attached to NK-cell lytic granules via its tailpiece, as best visualized using platinum rotary electron microscopy, and is not present in large radial filaments, as determined by irregularly spaced fluorescent signal in STED imaging (Figure 6). Whereas the conditions used in the lytic granule isolation may remove some myosin IIA from the surface of the granule before evaluation, and the irregular molecule spacing observed in the STED images does not exclude the possibility of small clusters or minifilaments of myosin IIA associating with the granules, our results suggest that it is unlikely that large, ordered complexes of myosin IIA associate with NK-cell lytic granules. To our knowledge, this work represents the first use of STED ultra-resolution evaluation of NK-cell lytic granules. In addition, using both ultra-resolution techniques (STED and platinum rotary electron microscopy), we observed several myosin IIA molecules on each lytic granule, with an average of 22 molecules per granule circumference in STED images of isolated granules. This may reflect a requirement for groups of myosin IIA molecules to work in concert to direct lytic granule transport at the IS, because myosin IIA is not a conventionally processive motor. The S1943A-GFP–expressing cells also displayed a marked reduction in the amount of total myosin IIA with lytic granules (Figure 5C), suggesting that the nonphosphorylatable mutant acted as a dominant negative, preventing the association of endogenous myosin IIA with lytic granules. In these cells, the total amount of myosin IIA may therefore fall below a threshold required for myosin IIA function in lytic granule transport.
In light of these observations, we propose a model for the interaction of myosin IIA with NK-cell lytic granules (Figure 6F). S1943 phosphorylation by casein kinase II has been suggested to cause the myosin IIA nonhelical tailpiece to bend back onto the C-terminal portion of the rod domain, thus down-regulating its ability to form filaments.27 Because the tailpiece is also the location of lytic granule binding, in NK cells, S1943 phosphorylation could additionally expose a binding site for lytic granules that is unavailable in the unphosphorylated protein. Therefore, constitutive phosphorylation of a pool of myosin IIA at S1943 would ensure that myosin IIA could interact with lytic granules. In the case of the 1933x mutation, the truncated tailpiece may allow for weak binding of myosin IIA to lytic granules. In the present study, the association of 1933x myosin with lytic granules was less than previously observed,15 which is likely because of a more physically vigorous granule isolation method, which further substantiated a weak interaction between 1933x and lytic granules. Because the truncated myosin molecules have been shown to bind both actin and the granules, at least under certain conditions, they may act to stabilize the granules at the surface of actin, allowing for them to eventually reach the plasma membrane. In the case of the S1943A point mutation, however, an unphosphorylatable myosin tailpiece would remain in a conformation in which the binding site for lytic granules was obscured. This would prevent any interaction with the lytic granules—potentially even in the subset of myosin IIA molecules in our cell lines, in which one heavy chain is wild-type and the other is mutated, causing a greater reduction in total myosin IIA quantity on the granule. Therefore, the presence of S1943A myosin would be more disruptive than 1933x myosin, particularly if groups of molecules are required to work together, either in single-molecule form or in small clusters, to promote motility of lytic granules across actin. This mechanism would explain the observed greater reduction in cytotoxicity in the YTS-S1943A-GFP cells compared with the 1933x-GFP cells, because the lytic granules in the former would be less capable of stably interacting with actin in vivo.
Whereas interacting with organelles in a single-molecule conformation represents an unconventional role for myosin IIA, previous reports have suggested a similar mechanism in other systems. An interaction of myosin II with secretory vesicles has been demonstrated in diverse cell types.42-47 Furthermore, in clam oocyte extracts and Drosophila neurons, inhibition of myosin II activity reduces the motility of vesicles along actin,45,47 suggesting that myosin II can direct the motility of vesicles as cargo along actin filaments. Although myosin IIA is not conventionally thought to be a processive motor, a recent study has suggested that myosin IIB may be processive across short distances.48 Therefore, it is possible that once lytic granules are delivered to the IS, myosin IIA could direct the motility of lytic granules across short stretches of actin to the plasma membrane. In addition, motor molecules that lack processivity as a single unit can still direct processive motion of cargo if enough motors exist on the cargo that at least one is in contact with the track at all times.49 Our data indicate that multiple myosin IIA molecules exist on each lytic granule (Figure 6A-E), and therefore the cooperation of these molecules may be sufficient to direct lytic granules across actin at the IS. Whereas the specific linkage between individual myosin IIA molecules and granules requires further study, the present results define a new role for myosin IIA in the immune system and in governing NK-cell function. In particular, we found that single-molecule, tail-based associations with granules and myosin IIA tail phosphorylation are essential for accessing NK-cell cytotoxicity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Geoff Daniels for assistance with STED imaging and members of the Orange laboratory for helpful discussions.
This work was supported by the National Institutes of Health (grant R01-AI067946 to J.S.O., training grant T32-GM07229 to K.B.S., and training grant 5T32-AR007442-24 to G.D.R.).
National Institutes of Health
Authorship
Contribution: K.B.S. designed and performed the experiments and wrote the manuscript; E.M.M. and G.D.R. performed the experiments; A.D. and J.A.M. performed sequencing of patients with MYH9-RD; A.P., R.F., and J.B.B. provided samples from patients with MYH9-RD; and J.S.O. initiated and directed the research, designed the experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jordan S. Orange, MD, PhD, Children's Hospital of Philadelphia, Division of Immunology, Abramson Research Center 907A, 3615 Civic Center Blvd, Philadelphia, PA 19104; e-mail: orange@mail.med.upenn.edu.

![Figure 1. Effect of naturally occurring mutations in MYH9 on human NK-cell cytotoxicity. (A) Schematic depicting the major functional regions of a myosin IIA molecule, indicating the position of MYH9 mutations in patients whose NK cells were used in this study. (B) Cytotoxic activity of negatively selected, purified ex vivo human NK cells from patients with mutations in the head (S96L, patient 1 [P1]), S2 (T1155I, P2), rod (R1400W, P3; D1424N, P4-P6), and tail (5779delC, P7) domains of MYH9 was compared with control donors in 51Cr-release assays against K562 target cells. Results from 1933x patients were published previously15 and are shown for comparison. Results are listed as lytic units required for 10% lysis of target cells (LU10). Results are adjusted for NK-cell purity of the isolated cells, and patient cell LU10 is normalized to control for each assay. For 1933x, when > 1 patient was analyzed, the mean ± SD is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/22/10.1182_blood-2011-03-344846/4/m_zh89991181320001.jpeg?Expires=1769095819&Signature=iAB5RJZgFCE40ud~MpC5KgJuvev~Kn~SrCD41W28cPOp--krEbVaX7~W5IkMre~sGNG0zdCwlpKArdJw15mJELi-kMZpYlzGbfVyVFhJbszEhjHeRaNkJGQ3MZNKxWXe0PK5Krpy4kxUVuMA3BzYERucOpAhbdmf3SQ1EeiLG0IQKF2NhvzEZTDQyUEXmyOSbKWu9WV3vfmhbnhHDkRbUvZqHAes29eS5Xmc2AwRztm2EcLcLkokCF5iNzY~R2Obmq74IQwalczXIsW348KcaO2kPSP2iASEESds335vrWGL4aY2cN2hbPEcfaUHxwd1ALqaDm2ZzNYFDxxpLzp5qQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal