Abstract
HIV infection is characterized by immune system dysregulation, including depletion of CD4+ T cells, immune activation, and abnormal B- and T-cell responses. However, the immunologic mechanisms underlying lymphocytic dysfunctionality and whether it is restricted to immune responses against neo antigens, recall antigens, or both is unclear. Here, we immunized SIV-infected and uninfected rhesus macaques to induce immune responses against neo and recall antigens using a Leishmania major polyprotein (MML) vaccine given with poly-ICLC adjuvant. We found that vaccinated SIVuninfected animals induced high frequencies of polyfunctional MML-specific CD4+ T cells. However, in SIV-infected animals, CD4+ T-cell functionality decreased after both neo (P = .0025) and recall (P = .0080) MML vaccination. Furthermore, after SIV infection, the frequency of MML-specific antibody-secreting classic memory B cells was decreased compared with vaccinated, SIV-uninfected animals. Specifically, antibody-secreting classic memory B cells that produced IgA in response to either neo (P = .0221) or recall (P = .0356) MML vaccinations were decreased. Furthermore, we found that T-follicular helper cells, which are essential for priming B cells, are preferentially infected with SIV. These data indicate that SIV infection results in dysfunctional T-cell responses to neo and recall vaccinations, and direct SIV infection of T-follicular helper cells, both of which probably contribute to deficient B-cell responses and, presumably, susceptibility to certain opportunistic infections.
Introduction
HIV infection is characterized by progressive loss of CD4+ T cells, eventually resulting in opportunistic infections and acquired immunodeficiency syndrome (AIDS) and, in untreated individuals, death. During acute HIV infection, CD4+ T cells are massively infected and rapidly depleted from effector sites, particularly in mucosal tissues, and as infection progresses to chronic disease, there is a progressive, slow loss of peripheral CD4+ T cells.1-7 Furthermore, HIV specifically infects activated memory CD4+ T cells, and previous studies have demonstrated that the virus preferentially infects CD4+ T cells where the corresponding antigen is often present at high levels, such as HIV-specific and Mycobacterium tuberculosis–specific cells.4,8
Hence, the immunodeficiency and dysfunctional immune responses that occur after HIV infection are of particular concern with respect to their role in providing immunity against opportunistic infections. The effectiveness of common vaccines in inducing healthy immune responses among chronically HIV-infected individuals against subsequent infection is decreased.9 Indeed, the majority of currently effective vaccination approaches induce protective immunity against pathogens by induction of long-term antibody responses specific for the vaccinated antigen.10 These antibodies are produced mainly by 2 subsets of B cells, plasma cells that reside primarily in the bone marrow and long-lived memory B cells.11-13 Moreover, development of an effective B-cell response is reliant on helper CD4+ T-cell responses that promote antigen-specific B-cell differentiation and antibody class switching.14 Class switching by B cells from their constitutive expression and production of IgM to other antibody classes (ie, IgG and IgA) is essential to develop an effective immune response, because each type of immunoglobulin has specific functions and sites of activity.10 IgA may be of particular importance during HIV infection, because IgA plays a significant protective role at mucosal surfaces,10 and given the common mucosal routes of HIV exposure.15 Thus, the overall loss of IgA that is observed during HIV infection16,17 may play a role in driving virus replication at mucosal sites as well as susceptibility to opportunistic infections. Furthermore, during chronic HIV infection, many other aspects of B-cell immunity are altered, including B-cell hyperactivation and increased B-cell turnover, increased production of autoantibodies, hypergammaglobulinaemia, and increased B cell–related malignancies.18-25 Moreover, there are increased frequencies of B-cell populations such as immature memory B cells, activated memory B cells, and plasmablasts that are not typically present at high levels in uninfected individuals.19,26
The mechanisms underlying the observed B-cell abnormalities within HIV-infected individuals are unclear, but one possibility could be abnormal T-cell responses. Indeed, B-cell differentiation and antibody class switching are dependent on well controlled immunologic phenomena within lymph node (LN) germinal centers and a particular subset of germinal center–resident CD4+ T cells termed T-follicular helper (Tfh) cells.27,28 As HIV infects CD4+ T cells, infection of Tfh cells may, in part, underlie the abnormal B-cell responses observed in HIV-infected individuals. However, there are multiple aspects of T-cell immunity that are abnormal within chronically HIV-infected individuals. These include high frequencies of T cells with an activated phenotype, increased exhaustion and terminal differentiation of T cells, increased turnover of T cells, and decreased expression of multiple effector cytokines.7,29-33
These observed immunologic dysfunctions are thought to limit the ability of HIV-infected individuals to mount healthy immunologic responses to antigenic stimulation in vivo. This, in turn, may increase the susceptibility of HIV-infected individuals to opportunistic infections. Indeed, previous studies have shown altered B cell–mediated responses to neo and recall vaccination in HIV-infected individuals with less diverse antibody isotypes and antibody titers that wane more quickly than observed in HIV-uninfected individuals.34-36 The importance for viral replication in regard to these altered B-cell responses is highlighted by findings that HIV-infected individuals treated with antiretroviral therapies mount healthy antibody responses to neo and recall vaccination.37,38 Moreover, proliferative T-cell responses to neo antigens also are decreased in HIV-infected individuals.39 However, potential mechanisms underlying the HIV-associated decreased immunologic responses to antigenic stimulation in vivo remain unclear.
To evaluate possible effects of SIV on immunologic responses to neo and recall antigenic stimulation, we vaccinated 3 groups of rhesus macaques (RMs), divided according to vaccination relative to SIV infection, with the L major polyprotein MML (Leish-111f)40 given with the adjuvant poly-ICLC. MML was chosen as a vaccination for this protocol based on previous studies that have demonstrated that this vaccination approach results in robust CD4+ T-cell and B-cell responses.41,42 Furthermore, L major is the causative agent of cutaneous leishmaniasis,43 a common opportunistic infection in HIV-infected individuals.44-46
To dissect naive (neo) versus memory (recall) MML responses, RMs were split into 3 groups of 5 animals each, and animals were vaccinated for both neo and recall responses in the presence or absence of SIV (Table 1). We studied CD4+ T-cell functionality by measurement of effector functions in response to MML stimulation in vitro, and B-cell functionality by MML-specific antibody production. We further assessed whether either MML-specific CD4+ T cells in blood or Tfh cells in LNs were preferentially SIV infected in vivo. We found a prominent loss of functionality for both CD4+ T cells and B cells in SIV-infected animals that was associated with preferential SIV infection of LN-resident Tfh cells but not with preferential infection of MML-specific CD4+ T cells. Thus, these data indicate that both neo and recall immune responses are deficient in response to vaccination in SIV-infected RMs.
Study outline
| Group . | MML vaccine (prime) . | MML vaccine (boost) . | MML response . | SIVmac239 infection . | MML vaccine (prime) . | MML vaccine (boost) . | MML response . |
|---|---|---|---|---|---|---|---|
| 1 | Day 0 | Week 4 | Neo | Week 18 | Week 22 | Recall | |
| 2 | Day 0 | Week 4 | Week 8 | Neo | |||
| 3 | Day 0 | Week 4 | Neo | Week 14 | Week 18 | Week 22 | Recall |
| Group . | MML vaccine (prime) . | MML vaccine (boost) . | MML response . | SIVmac239 infection . | MML vaccine (prime) . | MML vaccine (boost) . | MML response . |
|---|---|---|---|---|---|---|---|
| 1 | Day 0 | Week 4 | Neo | Week 18 | Week 22 | Recall | |
| 2 | Day 0 | Week 4 | Week 8 | Neo | |||
| 3 | Day 0 | Week 4 | Neo | Week 14 | Week 18 | Week 22 | Recall |
Each group consisted of 5 RMs. Group 1 was given MML vaccines on day 0 and week 4, and neo MML responses were determined at week 6. MML was again given at weeks 18 and 22, and recall MML responses were determined at week 24. Group 2 was infected with SIVmac239 on day 0, and MML vaccines were given at weeks 4 and 8, and neo MML responses were determined at week 10 in SIV-infected animals. Group 3 was given MML vaccines on day 0 and week 4, and neo MML responses were determined at week 6. Group 3 was then infected with SIVmac239 at week 14, and MML vaccines were again given at weeks 18 and 22, and recall MML responses were determined at week 24 in SIV-infected animals.
Methods
Study animals
Fifteen RMs were split into 3 study groups, with 5 animals per group as follows: (1) neo MML vaccination and subsequent recall MML vaccination in SIV-uninfected animals, (2) neo MML vaccination in SIV-infected animals, and (3) neo MML vaccination in SIV-uninfected animals followed by SIV infection and subsequent recall MML vaccination (Table 1). Animals were vaccinated subcutaneously with a mixture of 50 μg of MML protein and 1 mg of poly-ICLC (Oncovir) at each vaccination time point (Table 1). RMs in groups 2 and 3 were infected with 3000 median tissue culture infective dose of SIVmac239 intravenously at the time points outlined in Table 1. Peripheral blood was used for all studies, except for cell-associated virus quantification of Tfh cells that was performed on lymphocytes isolated from LNs from chronically SIV-infected RMs. LN biopsies were processed as described previously.47 Animals were housed and cared in accordance with American Association for Accreditation of Laboratory Animal Care standards in accredited facilities, and all animal procedures were performed according to protocols approved by the Institutional Animal Care and Use Committees of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Cell separation
The RMs were maximally bled (250-450 mL, based on the weights of the animals) into Vacutainer CPT peripheral blood mononuclear cell preparation tubes with sodium citrate (BD Biosciences). After centrifugation, mononuclear cells were then washed with PBS and separated into T- and B-cell lymphocyte fractions. Specifically, B cells were negatively selected using PE-conjugated antibodies against CD3 (clone SP34-2; BD Biosciences), CD14 (clone M5E2; BD Biosciences), CD16 (clone 3G8; BD Biosciences), and CD66 (clone TET2, Miltenyi Biotec) followed by anti-PE MicroBeads (Miltenyi Biotec). Labeled cells were then run over LD magnetic columns (Miltenyi Biotec). The T-cell lymphocyte fraction was then collected from the column after B-cell depletion. Purities of the total B-cell fraction were typically more than 74%, with less than 7% T-cell contamination.
T-cell stimulation and flow cytometry
T cells were stimulated overnight (13-15 hours) with 20 μg/mL MML protein and 5 μl/mL anti-CD28 ECD (clone 28.2; Beckman Coulter), and after 1 hour of stimulation 1 μg/mL brefeldin A was added (Sigma-Aldrich). Cells were then washed and stained for appropriate surface antibodies, including anti-CD3 Alexa700 (clone SP34-2; BD Biosciences), anti-CD8 PacBlue (clone RPA-T8; BD Biosciences), anti-CD4 PE-Cy5.5 (clone OKT4; eBioscience), aqua LIVE/DEAD amine dye (Invitrogen), anti-CD95 PE-Cy5 (clone DX2; BD Biosciences), and anti-CD14 APC (clone M5E2; BD Biosciences). All samples were then permeabilized and fixed using CytoFix/Perm kit (BD Biosciences) and intracellularly stained to detect anti-IFNγ PE-Cy7 (clone 4S.B3; BD Biosciences), anti-TNFα FITC (clone MAb11; BD Biosciences), and anti-CD40L APC-eFluor780 (clone 24-31; eBioscience). Flow cytometric acquisition was performed on an LSRFortessa or FACSAria II cell sorter driven by the FACSDiva Version 6.0 software (BD Biosciences). Analysis of the acquired data was performed using FlowJo Version 9.0.2 software (TreeStar). We used a minimum threshold of 200 collected events for all analysis. Analysis and presentation of distributions were performed using SPICE Version 5.1 downloaded from http://exon.niaid.nih.gov.48 Comparison of distributions was performed using a Student t test, and a partial permutation test as described previously.48
Cell-associated SIV DNA quantification
The SIV infection frequency of MML-specific CD4+ T cells compared with naive, central memory, and effector memory CD4+ T cells was determined by flow cytometrically sorting each subset. Naive CD4+ T cells were defined as live, CD3+CD4+CD28+CD95− lymphocytes; memory CD4+ T cells were defined as live, CD3+CD4+CD28+CD95+ lymphocytes; effector CD4+ T cells were defined as live, CD3+CD4+CD28−CD95+/− lymphocytes; and memory/effector CD4+ MML-specific T cells were defined as TNFα+, and IL-2+, CD40L+, or IFNγ+.
T cells were sorted using an FACSAria II cell sorter and FACSDiva software (BD Biosciences). Sorted cells were then lysed using 25 μL of a 1:100 dilution of proteinase K (Roche Diagnostics) in 10mM Tris buffer. Quantitative PCR was performed using 5 μL of cell lysates per reaction. Reaction conditions were as follows: 95°C holding stage for 5 minutes, and 50 cycles of 95°C for 15 seconds followed by 60°C for 1 minute using the Taq DNA polymerase kit (Invitrogen). The sequence of the forward primer for SIVmac239 is GTCTGCGTCATYTGGTGCATTC. The reverse primer sequence is CACTAGYTGTCTCTGCACTATRTGTTTTG. The probe sequence is CTTCRTCAGTYTGTTTCACTTTCTCTTCTGCG. For cell number quantitation, monkey albumin was measured as described previously.49 For PCR, we used a StepOne Plus real-time PCR system (Applied Biosystems), and the analysis was performed using StepOne software (Applied Biosystems).
To characterize Tfh for cell sorting, cells isolated from LNs were washed twice and incubated with LIVE/DEAD fixable aqua dead cell stain (Invitrogen), CD197/CCR7 PE-Cy7, and CD195/CCR5 PE (clones 3D12 and 3A9, respectively; BD Biosciences). Cells were then stained for surface markers with antibodies to CD3 PacBlue (clone SP34-2; BD Biosciences), CD4 APC-H7 (clone L200; BD Biosciences), CD279/PD-1 PE-Cy5.5 (clone J105; eBioscience), CD278/ICOS FITC (clone C398.4A; BioLegend), CD95 PE-Cy5 (clone DX2; BD Biosciences), and CD28 ECD (clone CD28.2; Beckman Coulter). Cells were then washed, permeabilized (Cytofix/Cytoperm buffer; BD Biosciences), and stained intracellularly with fluorescent-conjugated monoclonal antibodies to CD152/CTLA-4 APC (clone BNI3; BD Biosciences) and Ki67 Alexa700 (clone B56; BD Biosciences). Live CD3+CD4+ lymphocytes were sorted for naive (CD28+CD95−CD4+ cells), central memory (CCR7+PD1−CD95+CD4+ cells), and Tfh cells (ICOS+CTLA−4+PD1+CCR7−CD95+CD4+ cells). Sorted cells were then lysed and quantitative real time-PCR was preformed as described in the preceding paragraph. Statistical analysis was performed using Prism Version 5 (GraphPad Software) using the Mann-Whitney t test.
B-cell fractionation
Memory B cells were separated into CD27+ (classic memory) and CD27− B-cell fractions by magnetic beads. Cells were separated into CD27+ and CD27− fractions by labeling the classic memory B-cell fraction with biotinylated anti-CD27 (clone O323; BioLegend), followed by anti-biotin MicroBeads (Miltenyi Biotec), and recovery of the unlabeled and labeled fractions with MS magnetic columns (Miltenyi Biotec). Purities of the CD27+ and CD27− fractions were typically more than 80%.
ELISpot analysis
The ELISpot assays were performed as described previously 18,19 with the following modifications: 96-well nitrocellulose filtration plates (Millipore) were coated overnight at 4°C with the following: 5 μg/mL each anti–human λ and anti–human κ (Rockland Immunochemicals), 5 μg/mL keyhole limpet hemocyanin control antigen (KLH; EMD Calbiochem) as a negative control, or 5 μg/mL MML protein. The number of MML-specific spots was adjusted for background by subtracting spots in the relevant KLH wells from those in the test wells after cell input normalization. Average KLH responses were as follows: 63 antibody-secreting classic memory B cells (cmASCs)/106 for IgG, 220 cmASCs/106 for IgM, and 51 KLH cmASCs/106 for IgA. After a 4-day incubation of CD27+ B cells with 1:10 000 Staphylococcus aureus Cowan (EMD Biosciences) and 2.5 μg/mL CpG-B, cells were collected, washed, counted, and transferred to the coated plates at dilutions ranging from 3 000 to 300 000 cells/well, depending on the coating antigen or antibody. The plates were then incubated at 37°C for 5 hours, followed by addition of biotinylated anti–human IgG (1:20 000; Jackson ImmunoResearch Laboratories), anti–human IgM (1:10 000; BD Biosciences), or anti–monkey IgA (1:10 000; Rockland Immunochemicals), and overnight incubation at 4°C. Plates were washed, incubated with substrate (ELISpot Blue; R&D Systems), and dried, and the spots were analyzed and counted with an automated CTL-BioSpot S5 UV analyzer and software (Cellular Technology).
Results
SIV infection results in loss of multifunctional CD4+ responses to MML vaccination
To evaluate CD4+ T-cell responses to either neo or recall MML vaccination in SIV-infected versus uninfected RMs, we used 3 groups of 5 animals. Group 1 animals were vaccinated with MML at weeks 0 (neo prime) and 4 (neo boost), and the consequent neo response was measured at week 6, followed by vaccinations for MML at weeks 18 (recall prime) and 22 (recall boost), and the recall MML response was measured at week 24, all in the absence of SIV infection (Table 1). Group 2 animals were first infected with SIV and then vaccinated with MML at weeks 4 and 8 after infection, and neo MML responses were measured in the presence of SIV at week 10 (Table 1). Group 3 animals were first vaccinated with MML at weeks 0 and 4, and the neo MML response was measured at week 6 in SIV-uninfected RMs. The group 3 animals were then infected with SIV at week 14, vaccinated again for MML recall at weeks 18 and 22, and the recall MML response was measured at week 24 in the presence of SIV infection (Table 1).
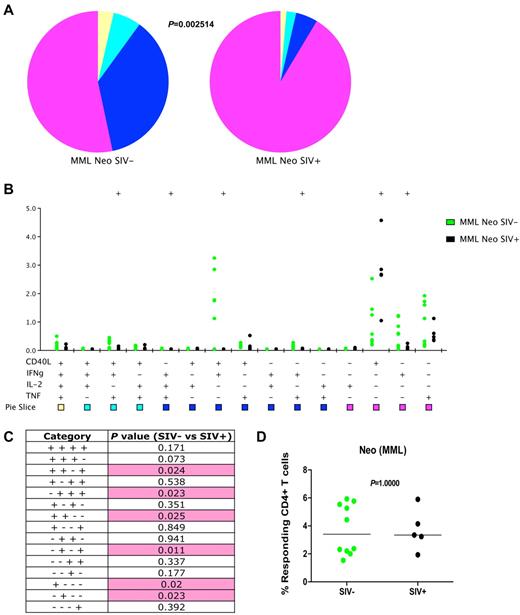
To study MML-specific CD4+ T-cell responses after neo or recall MML vaccination in SIV-infected versus uninfected animals, we stimulated T cells from vaccinated animals with MML protein and performed intracellular staining for CD40L, IFNγ, IL-2, and TNFα (representative stains are shown in supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We then assessed functionality based on the frequency of memory/effector CD4+ T cells that produced 4, 3, 2 (ie, were multifunctional), or 1 (ie, were monofunctional) functions. We observed robust, multifunctional T-cell responses to the neo MML vaccinations in SIV-uninfected RMs (Figure 1A-B; supplemental Figure 1A). However, in SIV-infected RMs, there was an overall loss of multifunctional CD4+ T cells (P = .0025; Figure 1A). Furthermore, several specific functional profiles were significantly decreased in SIV-infected compared with uninfected RMs, including CD40L+IFNγ+TNFα+ (P = .024), IFNγ+IL-2+TNFα+ (P = .023), CD40L+IFNγ+ (P = .025), IFNγ+TNFα+ (P = .011), and IFNγ, (P = .023; Figure 1B-C). However, in only one instance (CD40L+; P = .020) was there increased expression by CD; T cells in response to neo MML vaccination by SIV-infected RMs (Figure 1B-C). Of interest, despite the clear loss of multifunctional MML-specific CD4+ T cells in SIV-infected animals after neo MML vaccination, the total frequency of antigen-specific CD4+ T cells producing any combination of functions (1, 2, 3, and 4 functions) was equivalent (P = 1.000; Figure 1D), further demonstrating that although the frequency of responding CD4+ T cells is similar, the quality of the neo vaccination response after SIV infection is diminished. However, given that the SIV-infected animals have fewer CD4+ T cells overall, the SIV-infected animals also had lower total numbers of MML-specific CD4+ T cells that might decrease the immunologic responsiveness to antigen in vivo.
Loss of multifunctional MML-specific CD4+ T cells after SIV infection in neo-vaccinated animals. Flow cytometric analysis of MML-specific memory and effector (CD28+CD95+ and CD28−CD95+/−) CD4+ T cells 2 weeks after neo MML vaccinations by production of CD40L, IFNγ, IL-2, TNFα, or a combination. (A) Pie charts represent fraction of cells that have 4 (yellow), 3 (cyan), 2 (blue), or 1 (purple) function(s). P value calculated by partial permutation test. (B) Delineation of the 15 possible cytokine combinations is shown for SIV− (green) and SIV+ (black). Dots represent individual RM responses. (C) Student t test results between SIV− and SIV+ cytokine responses corresponding to panel B. Pink shading represents a significant P value less than .05. (D) Total frequency of cytokine producing memory and effector CD4+ T cells for SIV− (green) and SIV+ (black) in response to MML stimulation. P values represent partial permutation test results (A) or Student t test results (C). MML-specific responses from uninfected animals are based on data from group 1 and group 3 animals before SIV infection.
Loss of multifunctional MML-specific CD4+ T cells after SIV infection in neo-vaccinated animals. Flow cytometric analysis of MML-specific memory and effector (CD28+CD95+ and CD28−CD95+/−) CD4+ T cells 2 weeks after neo MML vaccinations by production of CD40L, IFNγ, IL-2, TNFα, or a combination. (A) Pie charts represent fraction of cells that have 4 (yellow), 3 (cyan), 2 (blue), or 1 (purple) function(s). P value calculated by partial permutation test. (B) Delineation of the 15 possible cytokine combinations is shown for SIV− (green) and SIV+ (black). Dots represent individual RM responses. (C) Student t test results between SIV− and SIV+ cytokine responses corresponding to panel B. Pink shading represents a significant P value less than .05. (D) Total frequency of cytokine producing memory and effector CD4+ T cells for SIV− (green) and SIV+ (black) in response to MML stimulation. P values represent partial permutation test results (A) or Student t test results (C). MML-specific responses from uninfected animals are based on data from group 1 and group 3 animals before SIV infection.
To determine whether SIV infection affected the functionality of memory or effector CD4+ T cells specific for recall antigens, we evaluated effector functions in response to MML stimulation after secondary (recall) MML vaccinations. We found that in SIV-infected RMs, there is an overall loss of multifunctional CD4+ T cells specific for MML (P = .0080; Figure 2A). Indeed, nearly every specific multifunctional profile was decreased in SIV-infected RMs compared with uninfected animals, including CD40L+IFNγ+IL-2+TNFα+ (P = .02), CD40L+IFNγ+IL-2+ (P = .01), CD40L+IL-2+TNFα+ (P = .01), IFNγ+IL-2+TNFα+ (P = .037), CD40L+IFNγ+ (P = .004), IFNγ+TNFα+ (P = .047), and IFNγ+ (P < .001; Figure 2B-C). Only 2 monofunctional profiles (CD40L+, P = .046 and IL-2, P = .027) were increased in SIV-infected compared with uninfected RMs in response to recall MML vaccination. Similar to MML neo responses, the overall frequency of MML-specific CD4+ T cells with any effector function was similar in SIV-infected or uninfected RMs after MML recall vaccinations (P = .3095), further indicating that SIV infection impacts the quality, not necessarily quantity, of memory T-cell responses.
Loss of multifunctional MML-specific CD4+ T cells after SIV infection in recall-vaccinated animals. Flow cytometric analysis of MML-specific memory and effector (CD28+CD95+ and CD28−CD95+/−) CD4+ T cells 2 weeks after recall MML vaccinations by production of CD40L, IFNγ, IL-2, TNFα, or a combination. (A) Pie charts represent fraction of cells that have 4 (yellow), 3 (cyan), 2 (blue), or 1 (purple) function(s). P value calculated by partial permutation test. (B) Delineation of the 15 possible cytokine combinations is shown for SIV− (green) and SIV+ (black). Dots represent individual RM responses. (C) Student t test results between SIV− and SIV+ cytokine responses corresponding to panel B. Pink shading represents a significant P value less than .05. (D) Total frequency of cytokine producing memory and effector CD4+ T cells for SIV− (green) and SIV+ (black) in response to MML stimulation. P values represent partial permutation test results (A) or Student t test results (C).
Loss of multifunctional MML-specific CD4+ T cells after SIV infection in recall-vaccinated animals. Flow cytometric analysis of MML-specific memory and effector (CD28+CD95+ and CD28−CD95+/−) CD4+ T cells 2 weeks after recall MML vaccinations by production of CD40L, IFNγ, IL-2, TNFα, or a combination. (A) Pie charts represent fraction of cells that have 4 (yellow), 3 (cyan), 2 (blue), or 1 (purple) function(s). P value calculated by partial permutation test. (B) Delineation of the 15 possible cytokine combinations is shown for SIV− (green) and SIV+ (black). Dots represent individual RM responses. (C) Student t test results between SIV− and SIV+ cytokine responses corresponding to panel B. Pink shading represents a significant P value less than .05. (D) Total frequency of cytokine producing memory and effector CD4+ T cells for SIV− (green) and SIV+ (black) in response to MML stimulation. P values represent partial permutation test results (A) or Student t test results (C).
Of note, there were also slight differences between neo and recall MML responses without accounting for SIV infection. In uninfected animals, there is a slight decrease in overall multifunctionality in recall MML responses compared with neo MML responses; however, a greater portion of the multifunctional responses after recall vaccination are 3 or 4 functions compared with neo vaccination, in which the multifunctional response is mostly comprised of 2 functions (P = .1988; supplemental Figure 2). Furthermore, in SIV-infected RMs there is a significant difference between overall neo and recall MML vaccination responses, with the most substantial contribution to this difference being CD40L+ cells, which are significantly increased after neo vaccination compared with recall vaccination (P = .0163; supplemental Figure 2).
MML-specific CD4+ T cells are not preferentially infected after MML vaccination
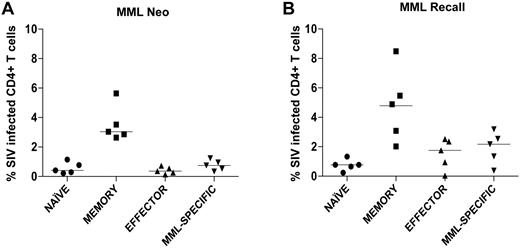
To determine whether the loss of multifunctional MML-specific CD4+ T cells, we observed after SIV infection was because of preferential SIV infection, we flow cytometrically sorted naive (CD28+CD95−), memory (CD28+CD95+), effector (CD28−CD95+), and MML-specific (TNFα+ and IFNγ+, IL-2+, or CD40L+) CD4+ T cells and assessed the level of SIV infection by quantitative real-time PCR. We found that MML-specific CD4+ T cells are infected after both neo (Figure 3A) and recall (Figure 3B) MML vaccination. However, MML-specific CD4+ T cells were not preferentially infected compared with other subsets of CD4+ T cells, even though these cells were of a memory (CD28+CD95+) phenotype (data not shown). Not surprisingly, however, we did observe that memory CD4+ T cells were preferentially infected compared with naive CD4+ T cells in all SIV-infected RMs (Figure 3). Thus, consistent with our data that the overall frequency of MML-specific CD4+ T cells are not reduced (Figures 1D and 2D), these data demonstrate that loss of functionality is not because of preferential SIV infection of responding CD4+ T cells.
MML-specific CD4+ T cells are not preferentially infected with SIV 2 weeks after MML vaccination. CD4+ T cells from SIV-infected RMs were sorted into 4 populations: naive (circles), memory (squares), effector (triangles), and MML-specific (inverted triangles) cells. Quantitative real-time PCR was used to determine the SIV infection frequency of each subset after neo MML vaccination (A) or recall MML vaccination (B). Horizontal bar represents median.
MML-specific CD4+ T cells are not preferentially infected with SIV 2 weeks after MML vaccination. CD4+ T cells from SIV-infected RMs were sorted into 4 populations: naive (circles), memory (squares), effector (triangles), and MML-specific (inverted triangles) cells. Quantitative real-time PCR was used to determine the SIV infection frequency of each subset after neo MML vaccination (A) or recall MML vaccination (B). Horizontal bar represents median.
Overall functionality of CD4+ T cells is reduced in SIV-infected RMs
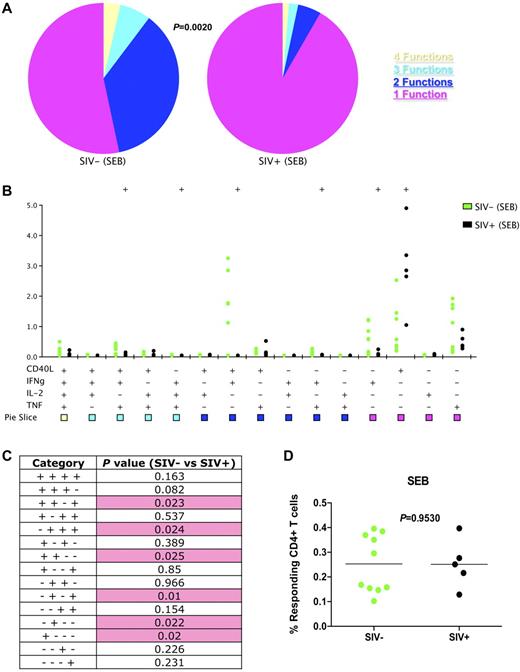
To determine whether the loss of functionality we observed in MML-specific CD4+ T cells extended to memory CD4+ T cells in general, we polyclonally stimulated T cells with the superantigen Staphylococcus enterotoxin B (SEB). Similar to MML-specific CD4+ T cells, the overall functionality of memory and effector CD4+ T cells was also decreased in SIV-infected animals compared with SIV-uninfected animals (P = .0020; Figure 4A). Specifically, after SIV infection there was a significant decrease in CD40L+IFNγ+TNFα+ (P = .023), IFNγ+IL-2+TNFα+ (P = .0024), CD40L+IFNγ+ (P = .025), IFNγ+TNFα+ (P = .01), and IFNγ+ (P = .022)-producing CD4+ T cells (Figure 4B-C). Also, similar to MML-specific cells, there was a significant increase of monofunctional CD40L+CD4+ T cells in response to SEB stimulation in SIV-infected RMs (P = .02; Figure 4B-C). Of interest, also similar to MML-specific CD4+ T cells, the total frequency of cytokine-producing cells was not decreased in SIV-infected RMs (P = .9530; Figure 4D). Thus, SIV infection does not necessarily result in a decreased frequency of functional cells, but a decreased simultaneous production of multiple effector functions by responding CD4+ T cells.
Overall CD4+ T cell functionality is decreased after SIV infection. Flow cytometric analysis of SEB-stimulated memory and effector (CD28+CD95+ and CD28−CD95+/−) CD4+ T cells by production of CD40L, IFNγ, IL-2, TNFα, or a combination. (A) Pie charts represent fraction of cells that have 4 (yellow), 3 (cyan), 2 (blue), or 1 (purple) function(s). P value represents partial permutation test. (B) Delineation of the 15 possible cytokine combinations is shown for SIV− (green) and SIV+ (black). Dots represent individual RM responses. (C) Student t test results between SIV− and SIV+ cytokine responses corresponding to panel B. Pink shading represents a significant P value less than .05. (D) Total frequency of cytokine producing memory and effector CD4+ T cells for SIV− (green) and SIV+ (black) RMs in response to SEB stimulation. P values represent partial permutation test results (A) or Student t test results (C). SEB responses from uninfected animals are based on data from group 1 and group 3 animals before SIV infection.
Overall CD4+ T cell functionality is decreased after SIV infection. Flow cytometric analysis of SEB-stimulated memory and effector (CD28+CD95+ and CD28−CD95+/−) CD4+ T cells by production of CD40L, IFNγ, IL-2, TNFα, or a combination. (A) Pie charts represent fraction of cells that have 4 (yellow), 3 (cyan), 2 (blue), or 1 (purple) function(s). P value represents partial permutation test. (B) Delineation of the 15 possible cytokine combinations is shown for SIV− (green) and SIV+ (black). Dots represent individual RM responses. (C) Student t test results between SIV− and SIV+ cytokine responses corresponding to panel B. Pink shading represents a significant P value less than .05. (D) Total frequency of cytokine producing memory and effector CD4+ T cells for SIV− (green) and SIV+ (black) RMs in response to SEB stimulation. P values represent partial permutation test results (A) or Student t test results (C). SEB responses from uninfected animals are based on data from group 1 and group 3 animals before SIV infection.
SIV infection leads to altered MML-specific B-cell functionality
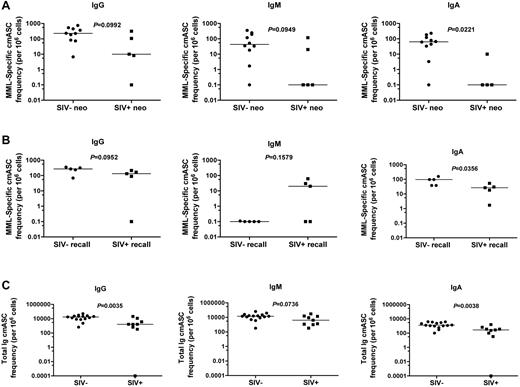
Helper CD4+ T cells are essential for promotion of effective, antigen-specific B cells, including class switching and the development of plasma cells. Given the loss of functionality we observed of CD4+ T cells in SIV infection, we next sought to determine whether SIV infection would be associated with altered B-cell responses, so we measured the frequencies of cmASCs in response to MML-specific stimulation and after polyclonal stimulation ex vivo. To do so, we sorted CD27+ (classic memory) B cells via magnetic bead separation, and we performed ELISpot analysis to determine the frequency of MML-specific IgG, IgM, and IgA cmASC responses after neo and recall MML vaccination. After neo MML vaccination, we observed a trend toward decreased frequencies of MML-specific IgG- (P = .0992) and IgM (P = .0949)-producing cmASCs in SIV-infected RMs (Figure 5A left and center). Furthermore, there was a significant decrease in MML-specific IgA-producing cmASCs (P = .0221; Figure 5A right) after neo MML vaccination in SIV-infected RMs. Thus, neo MML vaccination in the context of SIV infection results in decreased MML-specific cmASCs, specifically IgA-producing cmASCs, consistent with the finding that IgA levels are decreased in chronically HIV-infected individuals.16,17
MML-specific cmASCs are altered after SIV infection. ELISpot analysis of classic memory (CD27+) B-cell Ig responses (cmASCs). (A-B) Specific for MML or (C) total Ig (heavy and light chain, positive control). (A) Number (per 106 cells) of MML-specific cmASCs 2 weeks after neo MML vaccination for IgG (left), IgM (center), or IgA (right). (B) Number (per 106 cells) of MML-specific cmASCs 2 weeks after recall MML vaccination for IgG (left), IgM (center), or IgA (right). (C) Number (per 106 cells) of total Ig cmASCs 2 weeks after neo or recall MML vaccination for IgG (left), IgM (center), or IgA (right). SIV− RMs (circles) and SIV+ RMs (squares). P values represent Mann-Whitney U t test results, horizontal bar represents median. For one animal (CE5D), we did not have sufficient cells to perform the IgA assay.
MML-specific cmASCs are altered after SIV infection. ELISpot analysis of classic memory (CD27+) B-cell Ig responses (cmASCs). (A-B) Specific for MML or (C) total Ig (heavy and light chain, positive control). (A) Number (per 106 cells) of MML-specific cmASCs 2 weeks after neo MML vaccination for IgG (left), IgM (center), or IgA (right). (B) Number (per 106 cells) of MML-specific cmASCs 2 weeks after recall MML vaccination for IgG (left), IgM (center), or IgA (right). (C) Number (per 106 cells) of total Ig cmASCs 2 weeks after neo or recall MML vaccination for IgG (left), IgM (center), or IgA (right). SIV− RMs (circles) and SIV+ RMs (squares). P values represent Mann-Whitney U t test results, horizontal bar represents median. For one animal (CE5D), we did not have sufficient cells to perform the IgA assay.
Recall MML vaccination responses also were altered in SIV-infected animals. Similar to neo vaccination responses, MML-specific cmASC IgG responses also were slightly decreased in SIV-infected RMs (P = .0952; Figure 5B left). However, in contrast to neo vaccination responses, although we observed a low frequency of MML-specific IgM-producing cmASCs after recall vaccination in SIV-uninfected animals, in 3 of the recall MML-vaccinated RMs we observed a dramatic increase in MML-specific IgM cmASCs, and in 2 animals, there were no MML-specific IgM cmASCs detected (P = .1579; Figure 5B center). Furthermore, similar to neo vaccination, in response to recall vaccination there was a significant loss of MML-specific IgA-producing cmASCs after SIV infection (P = .0356; Figure 5B right). Thus, these data indicate that SIV infection results in compromised antigen-specific cmASC responses to recall MML vaccination, possibly associated with altered class-switching by B cells.
We next sought to determine whether plasma levels of MML-specific antibodies were altered in SIV infection. Thus, we developed an ELISA assay to determine the levels of IgG and IgA MML-binding antibodies in plasma. We found a trend toward a lower amount of IgG and IgA MML-specific antibodies in SIV-infected RMs compared with uninfected RMs after neo MML vaccination (supplemental Figure 3A,C). These data concur with our ELISpot analyses after neo MML vaccinations. However, these differences were not apparent after recall vaccination (supplemental Figure 3B,D).
To determine whether there is an overall loss of antibody production by CD27+ classic memory B cells after SIV infection in general, we polyclonally stimulated B cells with SEB and measured the frequencies of Ig (IgG, IgM, or IgA)–producing cmASCs. We found significant decreases in total IgG-producing cmASCs (P = .0035) and total IgA-producing cmASCs (P = .0038) as well as a trend toward decreased total IgM-producing cmASCs (P = .0736; Figure 5C). Thus, not only the MML-specific cmASC response but also the entire cmASC response is dysfunctional after SIV infection.
Tfh cells are preferentially infected after SIV infection
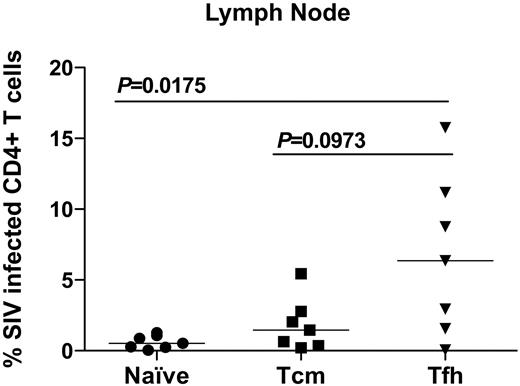
To unravel potential mechanisms underlying these observed immunologic dysfunctions, we initially compared CD4+ T-cell dysfunction and B-cell dysfunction with contemporaneous plasma viral loads. However, we found no associations between plasma viremia and dysfunction of either CD4+ T cells or B cells (data not shown). We therefore examined infection frequencies of LN-resident CD4+ T cells. In particular, specialized LN follicle-resident CD4+ T-helper cells, Tfh cells, are essential in promoting antigen-specific B-cell activation and class switching.28 Thus, we hypothesized that a potential mechanism underlying the dysfunction of classic memory B cells we observed after SIV infection was lack of CD4+ T cell help because of SIV infection of Tfh cells. To determine whether Tfh cells were SIV infected, we flow cytometrically sorted naive, central memory, and Tfh CD4+ T cells (defined as PD1+ICOS+CTLA−4+CCR7−, immunohistochemical analysis confirmed that PD1+CD4+ T cells reside in LN follicles; data not shown) from LNs of SIV-infected RMs and performed quantitative real-time PCR for levels of SIV DNA. We found that in LNs, there is a significant increase in frequencies of infected Tfh cells compared with naive CD4+ T cells (P = .0175), and we found a trend toward an increase compared with central memory CD4+ T-cell infection (P = .0973; Figure 6). Thus, Tfh cells, which are important for promoting B-cell responses to antigens, are infected after SIV infection, which may provide a mechanism underlying our observed B-cell dysfunction in SIV-infected RMs.
CD4+ Tfh cells are preferentially SIV-infected in LNs. CD4+ T cells from LNs of SIV-infected RMs were sorted into 3 populations: naive (circles), central memory (squares), and Tfh cells (inverted triangles). Quantitative real-time PCR was used to determine the SIV infection frequency of each subset. P values represent Mann-Whitney U t test results. Horizontal bar represents median.
CD4+ Tfh cells are preferentially SIV-infected in LNs. CD4+ T cells from LNs of SIV-infected RMs were sorted into 3 populations: naive (circles), central memory (squares), and Tfh cells (inverted triangles). Quantitative real-time PCR was used to determine the SIV infection frequency of each subset. P values represent Mann-Whitney U t test results. Horizontal bar represents median.
Discussion
Here, we have shown that MML-specific CD4+ T-cell responses after both neo and recall MML vaccination are significantly altered in SIV-infected RMs, with a significant loss of multifunctional CD4+ T cells and an increase in monofunctional (specifically CD40L+) CD4+ T cells. However, this loss of functionality was not attributed to preferential infection of responding CD4+ T cells by SIV. Moreover, we also found that overall CD4+ T-cell functionality in response to superantigen stimulation was decreased in SIV-infected RMs. Furthermore, MML-specific cmASCs were altered after SIV infection in response to neo and recall MML vaccination, with a specific, significant decrease in IgA cmASCs. We also observed an overall loss of total Ig (IgG, IgM, and IgA) production by cmASCs after SIV infection. Finally, we found that Tfh cells are preferentially infected by SIV; thus, infection and loss of these cells may underlie the B-cell dysfunctionality observed during SIV and HIV infection.
Our finding of decreased functionality among CD4+ T cells is consistent with previous data that has demonstrated that CD8+ T cells are less functional after HIV infection and that multifunctional cells are associated with nonprogressive HIV infection.50,51 Moreover, that CD4+ T-cell and CD8+ T-cell responses both manifest decreased functionality is consistent with our finding that direct SIV infection of the responding T cells is unlikely to lead to the observed phenomenon.
Previous studies have demonstrated that the degree of protection against L major in mice correlates with the degree of functionality of CD4+ T cells, specifically multifunctional CD4+ T cells that simultaneously produce IL-2, IFNγ, and TNFα.42 Here, we found that both neo and recall MML-specific as well as SEB-stimulated CD4+ T cells simultaneously expressing IL-2, IFNγ, and TNFα together was significantly decreased in SIV-infected RMs compared with uninfected RMs. This decreased ability for SIV-infected animals to induce this subset of T cells also may explain the high incidence of Leishmania infections that are observed in HIV-infected individuals.44-46 A better understanding of this particular subset of functionally defined CD4+ T cells may be important to understand why these cells are present at low frequencies in SIV-infected RMs and HIV-infected individuals. Furthermore, whether loss of these specific multifunctional cells contributes to other, non-Leishmania, opportunistic infections also should be investigated.
In addition, we observed variable but overall decreases in multifunctional CD4+ T cells, with concomitant increases in the frequencies of monofunctional, CD40L+, CD4+ T cells in response to either neo or recall MML vaccination or SEB. This is of interest because CD40L is a costimulatory molecule important for activation of antigen-presenting cells and is able to stimulate cytokine and chemokine expression by endothelial cells.52 Thus, increased CD40L production, expression by T cells, or both may play a role in the chronic immune activation observed during SIV and HIV infections.
B-cell responses via antibody secretion are essential for a protective immune response against pathogens. Here, we demonstrated a trend toward a decreased functionality of MML-specific as well as total Ig cmASCs in SIV-infected RMs, with the exception of an increase in IgM cmASCs in SIV-infected RMs after recall vaccination. This is of particular interest given that although antigen-specific IgM can provide protection, a healthy immune response should lead to increased frequencies of antibodies other than IgM after class switching occurs.14 Furthermore, the significant loss of IgA, which is fundamental for humoral mucosal immunity, in all cmASCs measured may play a role in the chronic dysfunction of gastrointestinal tract immunity during HIV and SIV infection. This is also consistent with previous studies demonstrating a loss of IgA in HIV-infected individuals,16,17 potentially an essential factor in the susceptibility to opportunistic infections arising in the mucosal immune system during HIV infection.
The mechanisms underlying the decreased adaptive immune responses to antigenic stimulation we observe within SIV-infected animals are probably multifaceted. However, one potential explanation is the preferential infection of Tfh cells. Indeed, follicular dendritic cells are thought to bind high numbers of infectious SIV virions.53 Given the proximity of Tfh cells to follicular dendritic cells within LN follicles, Tfh cells are poised for preferential infection. Preferential infection of Tfh cells could, in turn, hinder development of adaptive lymphocytes responding to antigenic stimulation. Indeed, Tfh cells are central for LN germinal center formation and express CD40L and multiple cytokines, such as IL-2, IL-4, IL-10, and IL-21, known to be important for orchestration of adaptive immune responses.54 Moreover, development of IgA-producing B cells has been shown to be dependent on IL-21 production by Tfh cells.54 Consistent with this premise, previous studies have shown decreased serum IL-21 and IL-21 production by T cells from HIV-infected individuals.55,56 Although we found no difference in expression of IL-21 among Tfh cells from LNs of SIV-uninfected and SIV-infected RMs, preferential infection of Tfh cells could alter the overall functionality of these cells, thereby potentially affecting the ability to mount appropriate adaptive immune responses.
We show here that SIV infection results in loss of functionality of both CD4+ T cells and classic memory B cells that may be associated with preferential infection of Tfh cells in LNs. These findings may help explain why opportunistic infections arise after HIV infection and may provide insight into avenues of therapeutic approaches designed to increase specific functionality of immune responses and protect HIV-infected individuals against opportunistic infections and AIDS.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Heather Cronise-Santis, JoAnne Swerczek, Richard Herbert, and all the veterinary staff at the NIH animal center. They thank the Cleveland Immunopathogenesis Consortium/Bad Boys of Cleveland for advice and helpful discussions.
This study was supported by the Intramural National Institute of Allergy and Infectious Diseases, National Institutes of Health program.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does it mention of trade names, commercial products, or organizations imply endorsement by the US Government.
National Institutes of Health
Authorship
Contribution: N.R.K. participated in study concept and design, acquisition, analysis and interpretation of data, statistical analysis, and drafting of the manuscript; C.L.V. participated in study concept and design and acquisition and analysis of data; R.M.L. participated in acquisition and analysis of data; L.A.C. participated in acquisition of data; J.H. participated in planning and interpretation of data; P.A.D. provided material support; S.L.M. analyzed and interpreted data and provided material support and intellectual contributions; J.D.E. participated in planning and interpretation of data; R.A.S. provided material support and contributed to study concept and design; and J.M.B. contributed to study concept and design, provided material support, and assisted in interpretation of data and study supervision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jason M. Brenchley, Program in Barrier Immunity and Repair and Laboratory of Molecular Microbiology, NIAID/NIH, 9000 Rockville Pike, Bldg 4, Rm 301, Bethesda, MD 20892; e-mail: jbrenchl@mail.nih.gov.
References
Author notes
N.R.K. and C.L.V. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal