In this issue of Blood, Li and colleagues demonstrate the transfer of leukemia and pathogen-specific immunity to murine bone marrow transplantation (BMT) recipients by vaccinating donors to recipient minor histocompatibility antigens (HAs) and subsequently transplanting a T-cell pool restricted to donor memory cells.1
They use a number of novel reagents to study and manipulate GVL effects against defined class I–restricted minor antigens in experimental transplant systems. In their study, donor mice were immunized with relevant recipient minor antigens linked to a DC-targeting antibody in conjunction with anti-CD40 antibody as an adjuvant. A number of months later, memory CD8 T cells were purified and transplanted with T cell–depleted donor bone marrow. Their data eloquently demonstrate that this approach leads to marked expansion of transferred memory T cells after BMT. This occurs in a CD4-independent fashion and significantly augments GVL against hematopoietic-restricted recipient minor HAs. The effect required cognate expression of the antigen on the leukemia cell and surprisingly induced little GVHD even if the antigen was ubiquitously expressed in the recipient. Interestingly, however, the GVL effect was largely lost when the antigen used in vaccination was ubiquitously expressed in the recipient. Critically, the approach allowed the concurrent transfer of immunity to other nominal (eg, pathogen-specific) antigens.
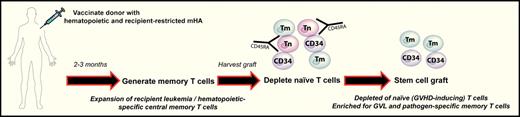
Donor vaccination to minor HA as a potential strategy to separate GVHD from GVL and pathogen-specific immunity after BMT. Donors are vaccinated with recipient minor Histocompatibility Antigens (mHA) to generate memory T cells (Tm). Stem cell grafts are subsequently harvested and naive T cells (Tn) depleted such that only stem cells (CD34+) and memory T cells with specificity for recipient hematopoiesis (ie, leukemia) and pathogens are transferred.
Donor vaccination to minor HA as a potential strategy to separate GVHD from GVL and pathogen-specific immunity after BMT. Donors are vaccinated with recipient minor Histocompatibility Antigens (mHA) to generate memory T cells (Tm). Stem cell grafts are subsequently harvested and naive T cells (Tn) depleted such that only stem cells (CD34+) and memory T cells with specificity for recipient hematopoiesis (ie, leukemia) and pathogens are transferred.
So how might this be relevant to clinicians and patients? This study establishes a relatively straight forward approach to separate GVHD from GVL and pathogen-specific immunity. It obviates the need for complex and expensive genetic and/or in vitro engineering of donor cells to introduce a T-cell receptor with specificity for leukemia (eg, via CD19 or leukemia-restricted antigens)2,3 and/or important pathogens (eg, to CMV or EBV).4 In the scenario suggested by Li et al, the selection of donor memory T cells enriches for pathogen-specific immunity already established within the donor (from prior pathogen challenges) while excluding naive T cells that appear to be critical for the induction of GVHD.1 Combining this approach with vaccination of the donor to hematopoietic and recipient-specific minor HAs allows the concurrent selection of leukemia-restricted donor T cells (see figure).
As always, there are a number of potential limitations to this approach. First, the identification of sufficient numbers of minor HAs with hematopoietic and recipient restriction is central to the feasibility of this strategy. However, significant developments are occurring in this field5 and minor HA-specific donor T cells are being generated in vitro and transferred into patients with demonstrable GVL activity.6 Second, the scenario requires vaccination of healthy donors to minor HAs and thus any risk of invoking autoimmune responses would have to be carefully considered and excluded. There may also be time limitations because this would need to be carried out some months before transplantation to allow the generation of central memory responses. Finally, formal demonstration that memory T cells fail to introduce GVHD in humans (like mice) is awaited from current clinical trials that use magnetic depletion of naive CD45RA+ donor T cells. The technology for the specific depletion of naive T cells will thus be a significant consequence of these studies.
After many decades, it appears we are slowly but surely moving toward the ability to separate GVHD and GVL responses. The current study provides experimental proof of principle of another approach to achieve this goal.
Conflict of interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal