Abstract
Advanced follicular lymphomas (FL) are incurable with conventional chemotherapy and there is no consensus on the best treatment approach. The SWOG cancer research cooperative group and Cancer and Leukemia Group B (CALGB) compared the safety and efficacy of two immunochemotherapy regimens for FL in a Phase III randomized intergroup protocol (S0016) that enrolled 554 patients with previously untreated, advanced stage FL between 3/1/2001 and 9/15/2008.
Pts were eligible if they had advanced stage (bulky stage II, III or IV) evaluable FL of any grade (1, 2, or 3) and had not received any prior therapy. In one arm (CHOP-R), patients received 6 cycles of CHOP chemotherapy (750 mg/m2 cyclophosphamide, 50 mg/m2 doxorubicin, 1.4 mg/m2 vincristine, and 100 mg prednisone daily for 5 days) at 3 week intervals with 6 doses of rituximab anti-CD20 antibody (375 mg/m2 on days 1, 6, 48, 90, 134 and 141 according to the schedule described by Czuczman et al.[J.Clin.Oncol 17:268, 1999]). In the second arm of the protocol, patients received 6 cycles of CHOP, followed by a dosimetric infusion of tositumomab/iodine I-131 tositumomab and then 1–2 weeks later a therapeutic infusion of I-131-tositumomab labeled with sufficient I-131 (median 85 mCi) to deliver a total body dose of 75 cGy (CHOP-RIT). The study was designed to have 86% power to detect a hazard ratio (HR) of CHOP-RIT to CHOP-R of 0.67 for 2 yr PFS based on a one-sided.021 level test (accounting for 3 interim analyses).
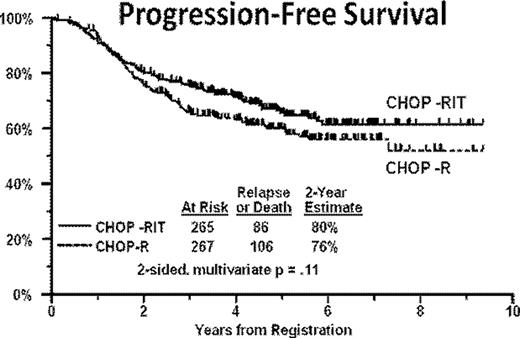
Of the 554 enrolled pts, 532 were eligible and 526 were evaluable for toxicity (263 on each arm of the protocol). Pt characteristics (age, sex, race, stage, beta 2 microglobulin level, tumor bulk, B symptoms) were well-balanced in the two arms of the protocol. In general, both regimens were well-tolerated (Table I). Median follow-up time among patients still alive is 4.9 years. One hundred and six of 267 eligible pts on the CHOP-R arm have progressed or died compared to 86 of 265 eligible pts on the CHOP-RIT arm. The 2 year estimate of PFS was 76% on the CHOP-R arm and 80% on the CHOP-RIT arm (Figure 1). In multivariate Cox regression adjusting for the stratification factor (serum beta-2 microglobulin level), the hazard ratio for CHOP-RIT vs. CHOP-R was 0.79 (95% CI: 0.60–1.05, p=.11 [2-sided] or p=.06 [1-sided]). Twenty-six of 267 pts on the CHOP-R arm have died compared to 40 of 265 eligible pts on the CHOP-RIT arm. The 2-year estimate of overall survival was 97% on the CHOP-R arm and 93% on the CHOP-RIT arm. In multivariate Cox regression adjusting for the stratification factor serum beta-2 microglobulin, the hazard ratio for CHOP-RIT vs. CHOP-R was 1.55 (95% CI: 0.95–2.54, p=.08 [2-sided]).
Toxicity and Efficacy of Treatment Arms on Protocol S0016
| . | CHOP-R . | CHOP-RIT . | P Values . |
|---|---|---|---|
| Evaluable for toxicity | N = 263 | N = 263 | |
| Grade 4 HematologicToxicity | 94 (36%) | 79 (30%) | .19 |
| Grade 4 Non-Hematologic Toxicity | 4 (1.5%) | 5 (1.9%) | 1.0 |
| Treatment Related Mortality | 1 (0.4%) | 4 (1.5%) | .37 |
| Second Malignancies | 23 (8.7%) | 22 (8.3%) | 1.0 |
| AML/MDS | 3 (1.1%) | 7 (2.7%) | .34 |
| Evaluable for response | N=263 | N=260 | |
| Overall Response Rate (CR + PR) | 224 (85%) | 223 (86%) | .90 |
| Complete Response Rate (including unconfirmed CR) | 108 (41%) | 120 (46%) | .25 |
| Response Assessment not possible (missing data) | 27 (10%) | 22 (8%) | .55 |
| Assessed for Survival | N=267 | N=265 | |
| 2 Yr Progression Free Survival (PFS) | 76% | 80% | p = 0.11 (2-sided) |
| HR=0.79 (95% CI: 0.60–1.05) | |||
| 2 Yr Overall Survival (OS) | 97% | 93% | p =0.08 (2-sided) |
| HR=1.55 (95% CI: 0.95–2.54) | |||
| . | CHOP-R . | CHOP-RIT . | P Values . |
|---|---|---|---|
| Evaluable for toxicity | N = 263 | N = 263 | |
| Grade 4 HematologicToxicity | 94 (36%) | 79 (30%) | .19 |
| Grade 4 Non-Hematologic Toxicity | 4 (1.5%) | 5 (1.9%) | 1.0 |
| Treatment Related Mortality | 1 (0.4%) | 4 (1.5%) | .37 |
| Second Malignancies | 23 (8.7%) | 22 (8.3%) | 1.0 |
| AML/MDS | 3 (1.1%) | 7 (2.7%) | .34 |
| Evaluable for response | N=263 | N=260 | |
| Overall Response Rate (CR + PR) | 224 (85%) | 223 (86%) | .90 |
| Complete Response Rate (including unconfirmed CR) | 108 (41%) | 120 (46%) | .25 |
| Response Assessment not possible (missing data) | 27 (10%) | 22 (8%) | .55 |
| Assessed for Survival | N=267 | N=265 | |
| 2 Yr Progression Free Survival (PFS) | 76% | 80% | p = 0.11 (2-sided) |
| HR=0.79 (95% CI: 0.60–1.05) | |||
| 2 Yr Overall Survival (OS) | 97% | 93% | p =0.08 (2-sided) |
| HR=1.55 (95% CI: 0.95–2.54) | |||
No statistically significant differences in PFS, OS, or serious toxicities are yet demonstrable with either regimen administered in this trial. However, PFS and OS are outstanding with either of the two regimens with median times to progression not yet reached for either treatment. Future studies will be needed to assess whether combining CHOP-R with RIT consolidation and with maintenance rituximab will confer additive benefit, as being evaluated in a follow-up trial (SWOG protocol S0801) that has recently completed accrual.
Press:Spectrum: Consultancy, Honoraria; Roche/Genentech: Consultancy, Honoraria, Research Funding. Off Label Use: Front-line use of I-131-tositumomab for consolidation therapy in 1st remission of follicular lymphoma. Friedberg:Genentech: Consultancy, Honoraria. Czuczman:Glaxo Smith Kline: Consultancy, Research Funding; Genentech: Consultancy, Honoraria. Kaminski:Glaxo Smith Kline: Patents & Royalties. Maloney:Genentech/Roche: Consultancy, Honoraria, Speakers Bureau; Glaxo Smith Kline: Consultancy, Honoraria, Speakers Bureau. Cheson:Glaxo Smith Kline: Research Funding. Fisher:Roche: Consultancy, Honoraria.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal