Abstract
Abstract 951
Gene expression profiling studies in Diffuse Large B cell Lymphoma (DLBCL) have described signatures enriched in genes representing the immune microenvironment. These signatures, together with the cell of origin classification have prognostic significance. However, a comprehensive functional understanding of the role of inflammation and the immune response in DLBCL is still lacking. The use of immunohistochemistry (IHC) to validate and elaborate upon molecular results is appealing. Our objective was to assess the cellular composition of the non-malignant population in DLBCL quantitatively and thoroughly in a single centre TMA of patients with DLBCL with corresponding clinical and survival data. Based on reported findings and known functional interactions a panel of antibodies were selected that included CD68, FOXP3 and TIA-1.
IHC was performed on TMAs from high quality formalin-fixed paraffin-embedded diagnostic tissue biopsies of DLBCL from 1977 to 2009. Triplicate cores were made from areas of representative tumour tissue, arrayed onto glass slides and stained for a panel of antibodies, aiming to characterize immune cell subsets. The TMA included tissue from 218 patients: 128 males, 90 females, with a median age of 55 (18-94) years; 23.2% high-int/high risk IPI; with median follow up of 3,1 years. 33% of patients were treated in the rituximab era. Absolute numbers of positive cells were counted across all intact cores using an automated image analysis system (Ariol), confirmed by histopathologists, and means calculated and corrected to a 1 mm2 area. A training-validation set method was used to generate optimal cutoff values discriminating prognostic groups and comparisons performed using the chi-square test.
Among nine antibodies examined, statistically significant prognostic information was provided using staining for FOXP3, TIA-1 and CD68. Using single cut-offs of positive cells/mm2 to define low and high marker density, two prognostic groups were defined for each marker. Patients with a more favourable prognosis had the highest cell density for FOXP3, TIA-1 and CD68.
| Marker (cutoff value high vs low) . | Overall Survival at 3yr (high vs low) . | Progression Free Survival at 3yr (high vs low) . | Disease Specific Survival at 3yr (high vs low) . |
|---|---|---|---|
| FOXP3 (450+ cells/mm2) | 64% vs 44% (p 0.005) | 55% vs 36% (p 0.001) | 69% vs 54% (0.01) |
| CD68 (900+ cells/mm2) | 58% vs 28% (p 0.023) | 50% vs 20% (p 0.05) | 66% vs 36% (p 0.005) |
| TIA-1 (2500+ cells/mm2) | 76% vs 45% (p 0.002) | 66% vs 41% (p 0.01) | 80% vs 56% (p 0.005) |
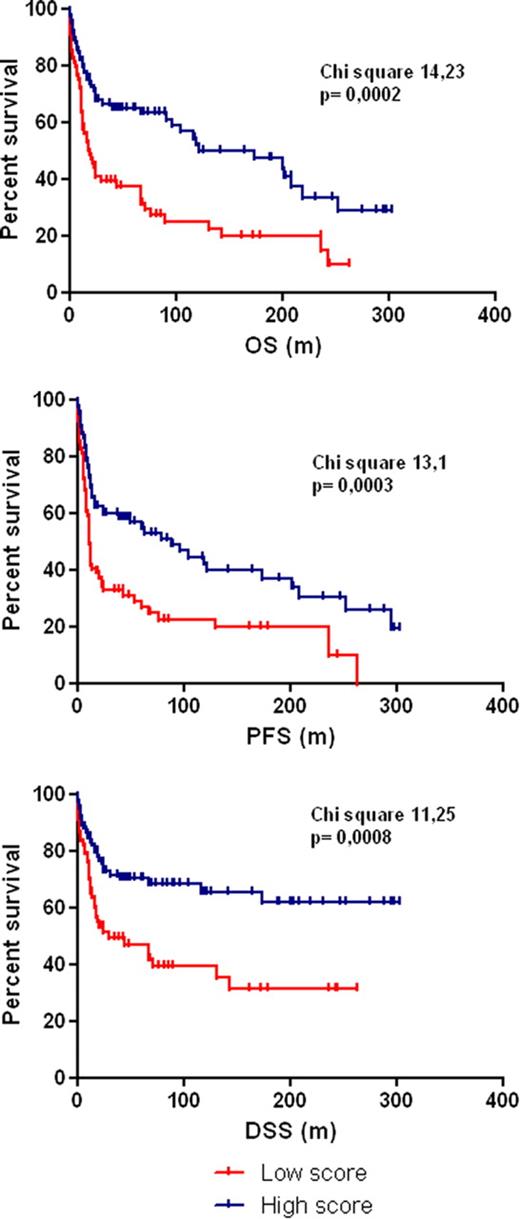
| Combined Score | 68% vs 39% (p 0.0002) | 60% vs 32% (p 0.0003) | 73% vs 49% (p 0.0008) |
| Marker (cutoff value high vs low) . | Overall Survival at 3yr (high vs low) . | Progression Free Survival at 3yr (high vs low) . | Disease Specific Survival at 3yr (high vs low) . |
|---|---|---|---|
| FOXP3 (450+ cells/mm2) | 64% vs 44% (p 0.005) | 55% vs 36% (p 0.001) | 69% vs 54% (0.01) |
| CD68 (900+ cells/mm2) | 58% vs 28% (p 0.023) | 50% vs 20% (p 0.05) | 66% vs 36% (p 0.005) |
| TIA-1 (2500+ cells/mm2) | 76% vs 45% (p 0.002) | 66% vs 41% (p 0.01) | 80% vs 56% (p 0.005) |
| Combined Score | 68% vs 39% (p 0.0002) | 60% vs 32% (p 0.0003) | 73% vs 49% (p 0.0008) |
A Cox regression model was developed, incorporating age, IPI, rituximab treatment and IHC score. The score results were validated as independent prognostic markers for OS, PFS and DSS, together with age, IPI (for OS and DSS) and rituximab (for PFS).
We confirm that there is heterogeneity in immune cell infiltration at diagnosis in DLBCL, and we provide correlative prognostic data to suggest a corresponding biological relevance. Attributing single cut-off values for immune cell markers is feasible, attractive as a clinical tool, and appears to provide robust prognostic information. In our single centre cohort we found that higher numbers of macrophages, Tregs and cytotoxic T cells correlate independently with improved patient survival. CD68 has been associated with an adverse prognosis in lymphoid malignancies but phenotypic diversity of macrophages may account for this study's finding and will be explored using macrophage subset specific markers.
Our multivariate analysis revealed that the IHC results have an independent impact on survival on this cohort of DLBCL patients. We are currently validating this approach in an independent TMA of R-CHOP treated patients. Combining these three different immune cell parameters allowed us to discriminate prognostic subgroups providing further evidence of the impact of the non-malignant immune cells in the biology of DLBCL and validating their role as potential therapeutic targets for intervention.
Gribben:Gilead: Honoraria; Mundipharma: Honoraria; GSK: Honoraria; Celgene: Honoraria; Roche: Honoraria; Pharmacyclics: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal