Abstract
Hodgkin Lymphoma (HL) is a highly curable malignancy affecting all age groups with a bimodal distribution with peaks between the 2nd and 3rd decade of life and at over 60 years of age. Children and adolescents with HL treated according to recent Pediatric Oncology Group (POG) regimens received dose dense, response-based chemotherapy with low dose radiation, decreasing both cumulative doses of chemotherapy and radiation. Treatment of adolescents and young adults (AYA) has not been consistent, with choice of adult vs. pediatric regimens depending on the referral pattern and institutional polices.
The German Cooperative group reported equivalent results in the AYA group vs. adults using an adult focused protocol that included high dose alkylator therapy and high dose radiation, and used that data to recommend adult therapy for AYA. We evaluated the outcome of Pediatric vs. AYA patients in two POG trials to assess the utility of pediatric regimens for AYA HL.
We retrospectively analyzed POG studies P9425 and P9426 to compare the survival rate of children (<15 year of age) and AYA (15 – 20 years of age) with HL.
P9425 included 216 patients (104 AYA) with intermediate (IB, IIA/IIIA1 with large mediastinal adenopathy or IIIA2) or high-risk (IIB, IIIB, IV) biopsy-proven classical HL. A response-based treatment approach administered doxorubicin, bleomycin, vincristine, etoposide, prednisone and cyclophosphamide (ABVE-PC) every 21 days. Rapid early responders (RER) to 3 ABVE-PC cycles received 21 Gy radiation to involved regions. Slow early responders (SER) received 2 additional ABVE-PC cycles before 21 Gy radiation. As previously published, this dose dense regimen resulted in excellent event-free and overall survival (EFS, OS) regardless of the risk assignment and early response. Five-year EFS 84%; 86% for RER, 83% for SER (P = 0.85). Five-year OS was 95%.
P9426 study included 255 patients, (99 AYA) with low risk (Stage IA, IIA and IIIA) biopsy-proven HL. Chemotherapy was a response-based approach that utilized doxorubicin, bleomycin, vincristine and etoposide (ABVE) every 28 days. RER to 2 DBVE cycles received 25.5 Gy involved field irradiation. SER received 2 additional cycles of ABVE followed by irradiation. The 5-year EFS for early stage HL under this protocol was 87.8%. 5-year OS was 97.6%.
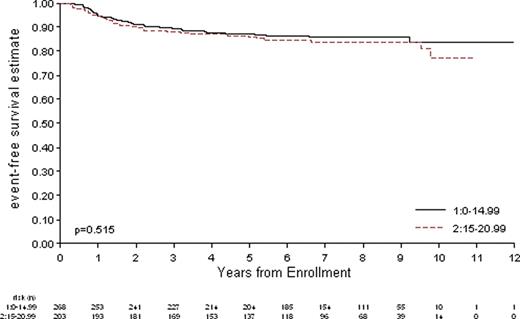
Of 471 eligible patients treated on POG 9425 and 9426, 203 were AYA. Table 1 shows patient characteristics. Male to female ratio was similar in both groups. The most common histology in both groups was nodular sclerosing. Mixed cellularity and lymphocyte predominant subtypes were more common in children less than 15 years of age. There was no difference in EFS for the younger vs. older patients.
Patient Characteristics
| . | <15 years N % . | ≥15 years N % . | Total N % . | Pr* . |
|---|---|---|---|---|
| STUDY | 0.042 | |||
| P9425 | 112 51.8% | 104 48.2% | 216 100% | |
| P9426 | 156 61.2% | 99 38.8% | 255 100% | |
| GENDER | 0.004 | |||
| Male | 180 67.2% | 110 54.2% | 290 61.6% | |
| Female | 88 32.8% | 93 45.8% | 181 38.4% | |
| HISTOLOGY | <0.001** | |||
| Nodular Sclerosis | 170 67.7% | 171 87.7% | 341 76.4% | |
| Mixed Cellularity | 57 22.7% | 16 8.2% | 73 16.4% | |
| Lymphocyte Predominant | 22 8.8% | 4 2.5% | 26 5.8% | |
| Lymphocyte Depleted | 2 0.8% | 4 2.1% | 6 1.4% | |
| RESPONSE | 0.57** | |||
| RER | 141 54.4% | 103 51.8% | 244 53.3% | |
| SER | 118 45.6% | 96 48.2% | 214 46.7% |
| . | <15 years N % . | ≥15 years N % . | Total N % . | Pr* . |
|---|---|---|---|---|
| STUDY | 0.042 | |||
| P9425 | 112 51.8% | 104 48.2% | 216 100% | |
| P9426 | 156 61.2% | 99 38.8% | 255 100% | |
| GENDER | 0.004 | |||
| Male | 180 67.2% | 110 54.2% | 290 61.6% | |
| Female | 88 32.8% | 93 45.8% | 181 38.4% | |
| HISTOLOGY | <0.001** | |||
| Nodular Sclerosis | 170 67.7% | 171 87.7% | 341 76.4% | |
| Mixed Cellularity | 57 22.7% | 16 8.2% | 73 16.4% | |
| Lymphocyte Predominant | 22 8.8% | 4 2.5% | 26 5.8% | |
| Lymphocyte Depleted | 2 0.8% | 4 2.1% | 6 1.4% | |
| RESPONSE | 0.57** | |||
| RER | 141 54.4% | 103 51.8% | 244 53.3% | |
| SER | 118 45.6% | 96 48.2% | 214 46.7% |
Based on Chi-square test
Chi Square test includes only available central review and early response data
Event-Free Survival in AYA Patients Compared to Children with HL Treated on P9425 and P9426
Event-Free Survival in AYA Patients Compared to Children with HL Treated on P9425 and P9426
The outcome of adolescents treated on P9425 and P2496 with dose dense, response-based treatment and reduced dose irradiation was not different from the outcome of children less than 15 years of age. The cumulative doses of alkylators, anthracyclines, and epipodophyllotoxins used in these pediatric protocols are below thresholds usually associated with significant long-term toxicity.
These data validate our preference for the treatment AYA and adolescents with HL using pediatric-focused therapy. This approach may reduce the risk for late adverse effects (cardiotoxicity, infertility, secondary malignancy) by limiting cumulative doses of alkylator agents, anthracyclines and radiation therapy. A focus on dose-limited regimens is critically important for younger patients (pediatric and AYA) who are expected to have long-term survival.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal