Abstract
Patients with thalassemic syndromes who require occasional or no blood transfusions are still at risk of iron overload (primarily from increased intestinal iron absorption caused by ineffective erythropoiesis) and associated serious complications. Iron chelation therapy (ICT) is the only option for decreasing iron burden, as phlebotomy is contraindicated due to anemia. The THALASSA 1-yr, randomized, double-blind, placebo-controlled study assessed efficacy and safety of deferasirox (DFX; Exjade®, once-daily oral iron chelator) in iron-overloaded patients with non-transfusion-dependent thalassemia (NTDT).
NTDT patients aged ≥10 yrs with liver iron concentration (LIC) ≥5 mg Fe/g dry weight (dw) and serum ferritin (SF) >300 ng/mL were enrolled and randomized 2:1:2:1 to starting doses of DFX/placebo 5 mg/kg/day or DFX/placebo 10 mg/kg/day. Patient inclusion/exclusion criteria were reported previously (Taher et al. Blood 2009;114(22):abst 5111). DFX dose could be doubled at 24 wks in patients with insufficient response (LIC >7 mg Fe/g dw and reduction <15% compared to baseline [BL]). The primary efficacy endpoint was change in LIC from BL to 1 yr calculated as least squares mean (LSM) based on ANCOVA adjusted for BL LIC. Other endpoints included change from BL in SF (LSM for quarterly SF changes), patients with LIC decrease ≥3 mg Fe/g dw or ≥30% from BL and DFX safety.
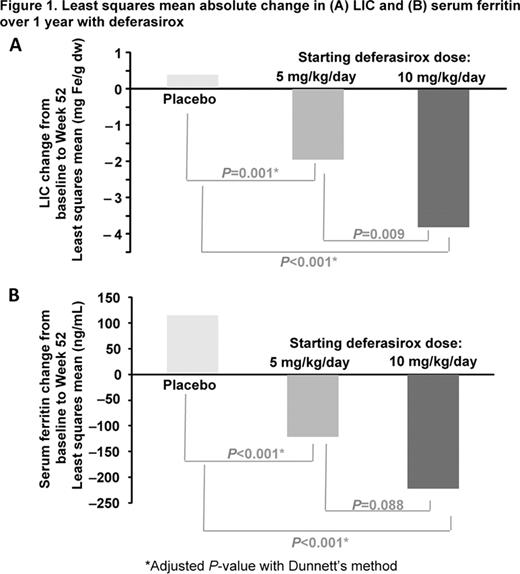
166 patients with β-thalassemia intermedia (n=95), α-thalassemia (n=22) or HbE/β-thalassemia (n=49) were randomized to starting DFX doses of 5 mg/kg/day (n=55) or matching placebo (n=28) and 10 mg/kg/day (n=55) or matching placebo (n=28). After 24 wks, 48.8% of all patients had a dose increase (47.3 and 45.5% in the DFX 5 and 10 mg/kg/day groups, respectively) resulting in an average actual dose (mean ± SD) of 5.6 ± 1.4 and 11.5 ± 2.9 mg/kg/day, respectively. At 1 year, LIC significantly decreased by –1.95 mg Fe/g dw (95% CI: –2.94, –0.96; BL: 13.11) and –3.80 mg Fe/g dw (95% CI: –4.76, –2.85; BL: 14.56) in the DFX 5 and 10 mg/kg/day groups, compared with an increase of 0.38 mg Fe/g dw (95% CI: –0.59, 1.34; BL: 15.94) in the placebo (Figure 1A); the difference between DFX 5 and 10 mg/kg/day groups vs placebo was significant (–2.33 mg Fe/g dw, P=0.001 and –4.18 mg Fe/g dw, P<0.001, respectively). At 1 yr, the percentage of patients with LIC decrease ≥3 mg Fe/g dw from BL was greater in the DFX 5 and 10 mg/kg/day cohorts (32.7% and 56.4%) compared to placebo (10.7%). In addition, more patients in DFX 5 and 10 mg/kg/day cohorts achieved LIC reduction ≥30% from BL (25.5% and 49.1%) compared to placebo (1.8%).
Mean serum ferritin significantly decreased at 1 yr: –121 ng/mL (95% CI: –203, –38) and –222 ng/mL (95% CI: –304, –140) for DFX 5 and 10 mg/kg/day groups (Figure 1B) while there was an increase in the placebo (115 ng/mL: 95% CI: 33, 196); difference vs placebo was significant (–235 ng/mL, P<0.001 and –337 ng/mL, P<0.001 for DFX 5 and 10 mg/kg/day, respectively).
148 (89.2%) patients completed 1 yr. In the placebo, DFX 5 and 10 mg/kg/day groups 8.9, 12.7 and 10.9% of patients discontinued, most commonly due to AEs (1.8, 3.6 and 5.5%, respectively). In addition, 1 patient (1.8%) in the placebo arm discontinued due to an abnormal lab value (low hemoglobin).
Overall AE rates were 80.4, 76.4 and 78.2% in the placebo, DFX 5 and 10 mg/kg/day groups, respectively. The most common investigator-assessed drug-related AEs in the overall placebo (n=56), DFX 5 and 10 mg/kg/day groups, respectively, were nausea (7.1, 5.5 and 7.3%), rash (1.8, 3.6 and 9.1%), diarrhea (1.8, 0 and 9.1%), headache (3.6, 3.6 and 1.8%) and upper abdominal pain (0, 3.6 and 1.8%). Serious AEs were reported in 14.3, 12.7 and 16.4% of patients in the placebo, DFX 5 and 10 mg/kg/day groups, respectively. 3 (5.5%) DFX-treated patients (DFX 10 mg/kg/day) had 2 consecutive serum creatinine level increases >33% above BL and >upper limit of normal (ULN). 1 patient in the placebo group had an ALT increase >5 × ULN and >2 × BL and 2 patients had an AST increase to >5 × ULN and > 2 × BL, 1 in the DFX 10 mg/kg/day and 1 in the placebo group.
This first randomized, placebo-controlled study evaluating ICT in NTDT patients confirms that these patients have high LIC despite moderately elevated SF, thus highlighting the need for ICT. Compared to placebo, 1-year DFX at starting doses of 5 and 10 mg/kg/day escalated to 10 and 20 mg/kg/day significantly reduced LIC and SF, along with a similar frequency of overall AEs.
Taher:Novartis: Honoraria, Research Funding. Off Label Use: Exjade is indicated for the treatment of chronic iron overload due to frequent blood transfusions. This abstract describes off-label use of Exjade in patients with non-transfusion-dependent thalassemia syndromes with iron overload. Porter:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Viprakasit:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kattamis:Novartis: Honoraria, Research Funding, Speakers Bureau. Chuncharunee:Novartis: Research Funding. Sutcharitchan:Novartis: Research Funding. Siritanaratkul:Novartis: Research Funding. Galanello:ApoPharma: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau. Karakas:Novartis: Honoraria. Lawniczek:Novartis: Employment. Ros:Novartis: Employment. Zhang:Novartis: Employment. Habr:Novartis: Employment. Cappellini:Novartis: Speakers Bureau.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal