Abstract
Abstract 898
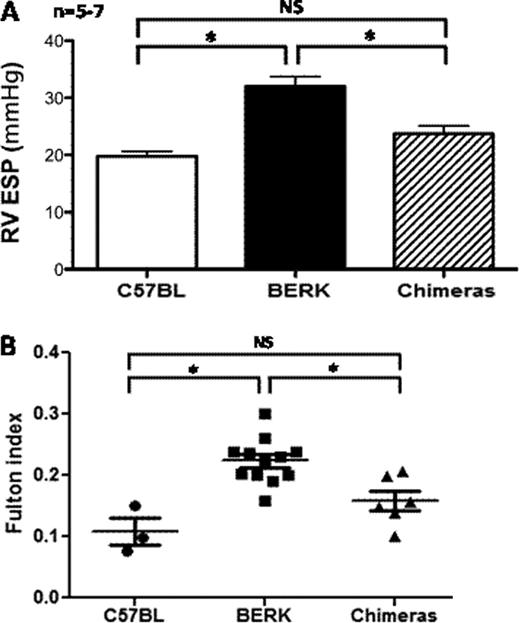
In sickle cell disease (SCD), mutant hemoglobin S polymerizes when deoxygenated, driving red blood cell (RBC)-dependent vaso-occlusion and hemolysis. These processes lead to platelet and hemostatic activation, pulmonary hypertension (PH) and vascular disease. Transgenic-knockout sickle (BERK) mice that express exclusively human α- and βS-globins mimic SCD in humans by displaying reduced nitric oxide (NO) bioavailability, impaired NO-mediated vascular reactivity and PH. Recently, the platelet α-granule protein thrombospondin-1 (TSP1) was found to be elevated in the plasma of patients with SCD and to potently inhibit physiologic NO signaling, via binding to the cell surface receptor CD47. We hypothesized that blocking the TSP1-CD47 interaction may restore NO signaling and prevent PH in BERK mice. To test this hypothesis we conducted a transplantation experiment to explore the repopulating potential of BERK bone marrow (BM) in lethally myeloablated CD47KO recipients and the impact of the CD47 null milieu on the PH phenotype. We harvested the BM from 5–6 months old BERK mice and transplanted it into irradiated (10 Gy) 8–9 weeks old CD47KO mice (n=9). All recipients survived transplantation and were terminally evaluated 4 months post transplantation. Mice underwent blood sampling for determination of engraftment by hemoglobin electrophoresis, evaluation of endothelial dependent arterial vasodilation by myography, full pulmonary hemodynamic assessment and measurement of right ventricular hypertrophy (RVH) using the Fulton Index (ratio of ventricular weights (right ventricle/left ventricle including septum). The chimeras had 98.3% (SD 0.6%) hemoglobin S, thereby demonstrating full donor chimerism. Segments of thoracic aortas from the chimeras were mounted on a myograph system and exposed to acetylcholine, a physiologic vasodilator that stimulates endothelial nitric oxide synthase (eNOS) activation. Concentration-response curves showed that the arterial segments from chimeras that lacked tissue CD47 had improved endothelial-dependent vasodilation, as evaluated by % relaxation in response to acetylcholine, as compared to arterial segments from BERK mice (P < 0.05). Hemodynamic data showed that the tissue CD47KO chimeras had lower right ventricular end systolic pressure (RV ESP) as compared to BERK mice (22 vs. 31 mm Hg, p<0.05). Conversely, their RV ESP did not significantly differ from historical control C57BL/6 mice (22 vs. 20 mm Hg, NS, panel A). Measurement of RVH (Fulton Index) similarly revealed that the chimeras were protected from RVH (p<0.05, panel B). Thus, despite the presence of sickle RBC, the absence of the TSP1-CD47 signaling axis improved endothelial-eNOS-NO signaling and reduced pulmonary pressures and RVH responses. These data demonstrate that BM from BERK mice successfully engrafts CD47KO mice, and that in the absence of the TSP1-CD47 axis endothelial and vascular function improves and PH is ameliorated. We now plan to validate these results in controlled experiments where BM from BERK mice is transplanted in CD47KO and C57BL mice as controls. We expect that unlike C57BL mice transplanted with BERK BM, CD47KO mice will be protected from the vascular complications of SCD, including PH. Close modal
Figure
legend: CD47KO mice transplanted with BERK BM (chimeras) show improved hemodynamics (Panel A) and less right ventricular (RV) hypertrophy as measured by the Fulton Index as compared to BERK mice (Panel B). * = statistically significant, NS = non significant, RV ESP = right ventricle end systolic pressure.
Figure
legend: CD47KO mice transplanted with BERK BM (chimeras) show improved hemodynamics (Panel A) and less right ventricular (RV) hypertrophy as measured by the Fulton Index as compared to BERK mice (Panel B). * = statistically significant, NS = non significant, RV ESP = right ventricle end systolic pressure.
Disclosures:
Isenberg:Vasculox, Inc.: Equity Ownership.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal