Abstract
Abstract 878
PTLD is a rare disorder affecting 1 to 10% of transplant recipients. Data from prospective trials is scarce. For more than 30 years, reduction of immunosuppression has been the basis of treatment despite generally low efficacy. The introduction of rituximab monotherapy has significantly improved remission rates in CD20-positive B-cell PTLD, but long-term disease control remains problematic. CHOP chemotherapy can achieve this goal, but is associated with a high lethal toxicity of 20% to 30%. Thus, we initiated an international multicenter phase II trial to investigate whether the subsequent application of 4 courses of rituximab and 4 cycles of CHOP-21 would improve the outcome.
Treatment-naive adult solid organ transplant recipients diagnosed with CD20-positive PTLD received 4 courses of rituximab (375 mg/m2) once a week followed by 4 weeks without treatment and 4 cycles of 3-weekly CHOP. In case of disease progression during rituximab monotherapy, CHOP was commenced immediately. Supportive therapy with G-CSF was mandatory and antibiotic prophylaxis was recommended. The primary endpoint was response and duration of response. Secondary endpoints were treatment toxicity and overall survival. Analysis was by intention to treat. This study is registered with EudraCT number 2005-000743-29.
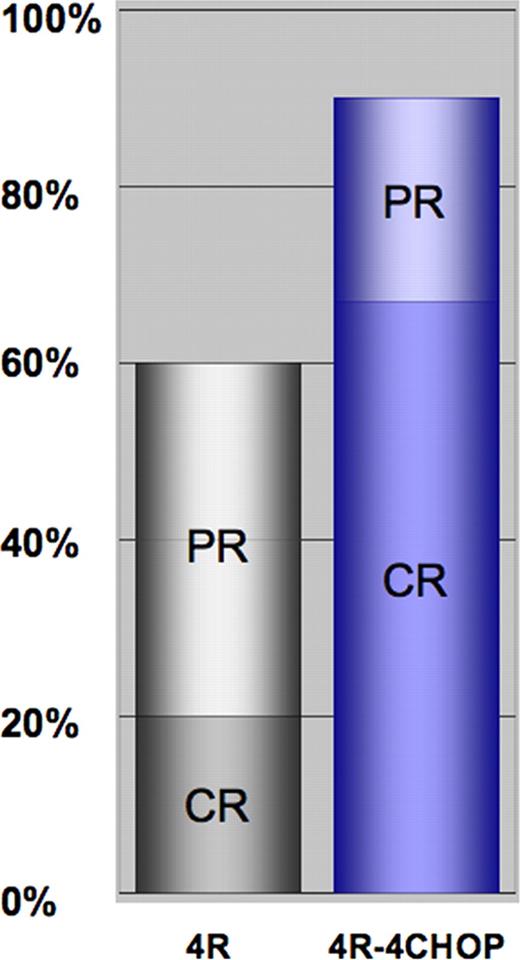
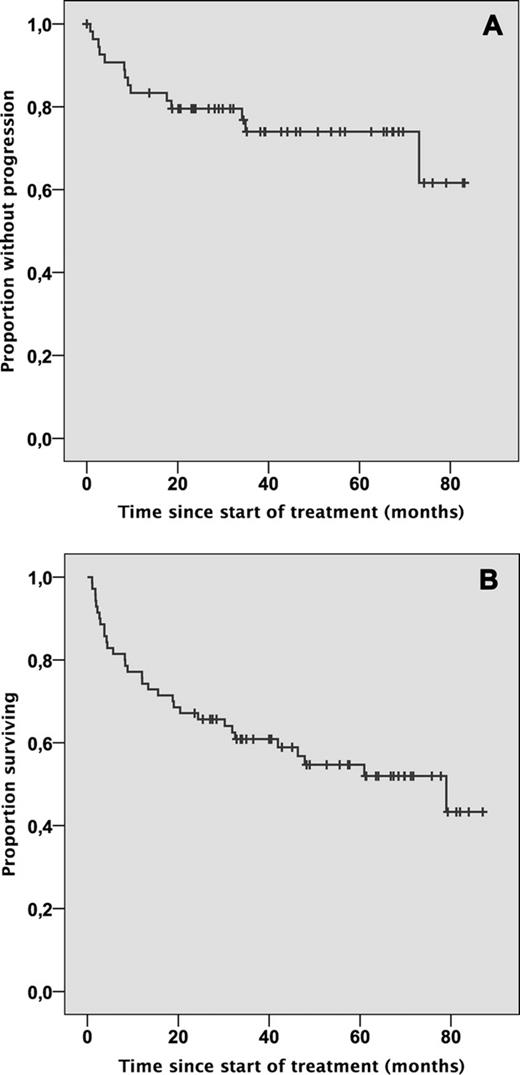
74 patients were enrolled between Jan. 2003 and Dec. 2007. 70 were allocated to treatment (4 excluded due to missing or withdrawn informed consent). Median age at diagnosis of PTLD was 53.3 years (range 16–74 years). The transplanted organs were kidney (29/70), liver (16/70), heart (14/70), lung (4/70), heart + lung (2/70), kidney + pancreas (4/70) and bone marrow (1/70, protocol violation). Median time of follow up was 5.1 years. PTLD occurred late (more than one year after transplantation) in 76%, with disseminated disease (Ann Arbor stage >II) in 74%. Histology was monomorphic in 84% and EBV-association was present in 44%. Patients with EBV-associated PTLD had a significantly different pattern of involvement and a significantly worse performance status. 66/70 patients (94%) received chemotherapy: two had died prior to CHOP and two were considered not eligible. Chemotherapy-induced grade 3/4 leukocytopenia occurred in 68% and grade 3/4 infections in 41% of patients. The planned dose of CHOP was reduced by more than 25% in 14% of patients. Dose reductions were significantly more frequent in EBV-associated PTLD and did correlate with more frequent grade 3/4 infections. Treatment-associated mortality of CHOP was 10.6% (7/66), the majority of which affected rituximab non-responders (5/7). The overall response rate was 90% (67% complete response, see figure 1). Median duration of remission for treatment responders is not yet reached and 74% are progression free at 3 and 5-years (figure 1A). Median overall survival was 6.6 years (figure 2B). EBV-association predicted time to progression (TTP) in adjusted Cox regression analysis (HR: 0.150, p=0.027), but patients with EBV- and non-EBV-associated PTLD demonstrated similar TTP intervals by univariate analysis. Notably, response to rituximab at interim staging predicted TTP (p=0.028) and OS (p=0.014), which was the reason to cease accrual to the protocol in 2007 and introduce risk-stratified sequential treatment (RSST).
CR and PR rates after four courses of rituximab monotherapy (4R) and after completion of therapy (4R-4CHOP).
CR and PR rates after four courses of rituximab monotherapy (4R) and after completion of therapy (4R-4CHOP).
A: Duration of response and B: overall survival. Analysis is by intention to treat. Median follow up is 5.1 years.
A: Duration of response and B: overall survival. Analysis is by intention to treat. Median follow up is 5.1 years.
Discussion This is the largest prospective trial in the field of PTLD published so far. Sequential treatment with rituximab and CHOP results in excellent disease control and overall survival in adults with PTLD, adding more than three years of life in comparison to historical results achieved with 1st-line rituximab monotherapy and 2nd-line (chemo)-therapy. TRM after sequential treatment is lower than historically reported for CHOP 1st-line therapy. The lower TRM from CHOP in rituximab responders versus non-responders supports the idea that it is a function of tumor burden. In conclusion, sequential treatment with 4 courses of rituximab followed by 4 cycles of three-weekly CHOP + GCSF provides an excellent standard of care for patients with both EBV-associated and non-EBV-associated B-cell PTLD failing to experience sustained response to upfront reduction of immunosuppression.
Trappe:AMGEN: Research Funding; Mundipharma: Research Funding; CSL Behring: Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding. Oertel:Roche: Employment, Equity Ownership. Leblond:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees. Salles:Roche and/or Genetech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genzyme: Membership on an entity's Board of Directors or advisory committees; Calistoga/Gilead: Membership on an entity's Board of Directors or advisory committees. Morschhauser:Roche/Genetech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Choquet:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal