Abstract
Abstract 847 FN2
FN2
Platelet activation may play a role in the pathogenesis of various complications related to sickle cell disease (SCD). Older studies of antiplatelet agents in reducing the frequency and severity of pain in SCD have been inconclusive. Prasugrel is a third generation thienopyridine antiplatelet agent, an oral P2Y12 ADP receptor antagonist that is FDA-approved for use in patients with acute coronary syndrome undergoing percutaneous coronary intervention. We undertook a study to determine the safety, pharmacodynamics and possible efficacy of prasugrel in adult patients with SCD.
This was a multicenter, randomized, double-blind phase 2 adaptive study design with a 2:1 prasugrel:placebo randomization ratio. Study drug was given once daily for 30 days. The primary endpoint was safety as measured by hemorrhagic events requiring medical attention. Secondary endpoints included pain frequency and severity assessed by pain diary and pharmacodynamic effects measured by VerifyNow” P2Y12 reactivity units (VN PRU) and vasodilator stimulated phosphoprotein (VASP) phosphorylation platelet reactivity index (PRI). If interim analysis of pharmacodynamic data revealed insufficient platelet inhibition in the first 16 patients randomized to 5 mg daily prasugrel, the dose was to be escalated to 7.5 mg.
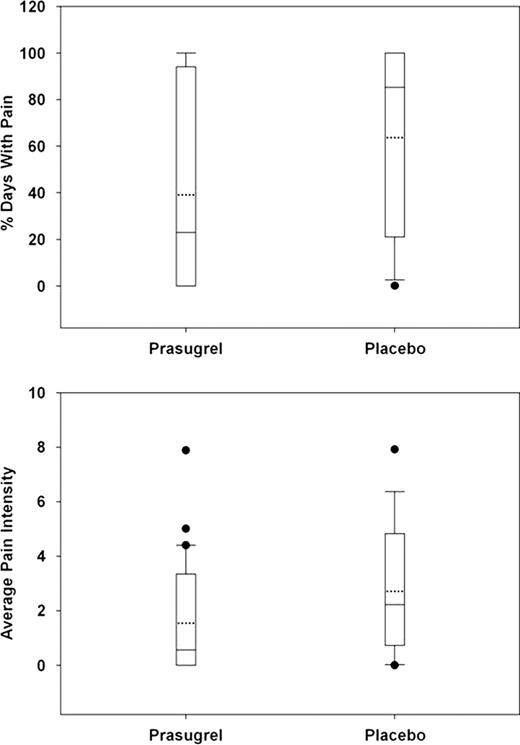
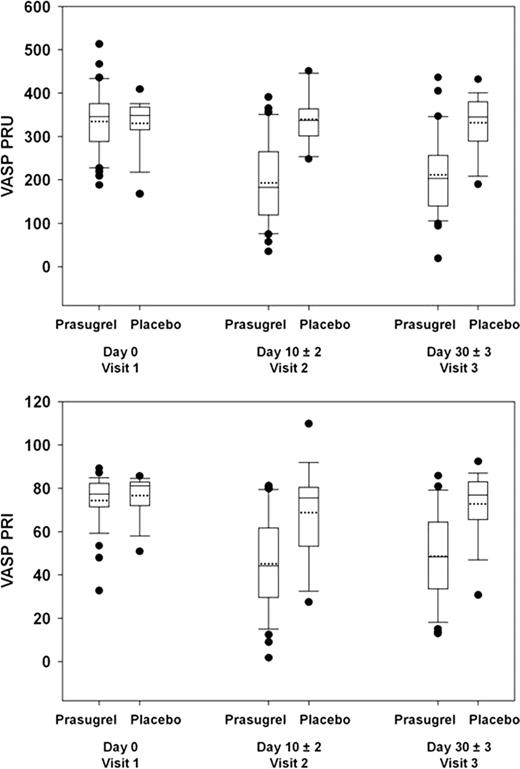
Forty-one patients were randomized to the prasugrel arm and 21 to placebo. Mean age was 32 years; 48% were female; 60% HbSS, 5% HbS/b0thalassemia, 10% HbS/b+thalassemia, and 24% HbSC (1 patient was found not to have SCD but to only have b-thalassemia trait). Eighteen patients (44%) in the prasugrel and 9 (43%) in the placebo arm were on hydroxyurea prior to study drug initiation. These characteristics were balanced between the treatment arms, as was baseline pain intensity (average pain score on a scale of 0 to 9 over 7 days prior to study drug). No hemorrhagic event required medical attention. Eight (20%) of patients in the prasugrel arm had hemorrhagic adverse events of which 6 (15%) were possibly related to study drug versus 1 (5%) in the placebo arm; all were mild except one moderate menorrhagia in a prasugrel-treated patient. There were numerical decreases in median and mean pain rate (% of days with pain) and intensity in the prasugrel arm compared to placebo but this did not reach statistical significance (Figure 1). The proportion of pain episodes requiring medical attention was also numerically lower: 23% prasugrel versus 37% placebo. There was measurable platelet inhibition as assessed by both VerifyNow” PRU and VASP PRI such that no dose escalation was necessary (Figure 2).
Whiskers represent 10th and 90th percentile; boxes represent 25th and 75th percentile; dotted line represent mean; center line represent median; solid dots represent outliers.
Whiskers represent 10th and 90th percentile; boxes represent 25th and 75th percentile; dotted line represent mean; center line represent median; solid dots represent outliers.
Whiskers represent 10th and 90th percentile; boxes represent 25th and 75th percentile; dotted line represent mean; center line represent median; solid dots represent outliers.
Whiskers represent 10th and 90th percentile; boxes represent 25th and 75th percentile; dotted line represent mean; center line represent median; solid dots represent outliers.
Prasugrel, 5 mg once daily for 30 days, had anticipated pharmacodynamic effect on platelets in adult patients with SCD, was well tolerated, and was not associated with serious hemorrhagic events. Despite the small size and short duration of this phase 2 study, there was a suggestion of decreased days with pain. These data provide a rationale for a Phase 3 study of prasugrel adequately powered to determine effects on relevant clinical outcomes such as pain rate, severity, and frequency of vaso-occlusive crisis.
Wun:Daiichi Sankyo Company, Ltd. and Eli Lilly and Company: Research Funding. Off Label Use: This abstract discusses prasugrel treatment in patients with sickle cell disease. Please see USPI for most up-to-date information. Krishnamurti:Daiichi Sankyo Company, Ltd. and Eli Lilly and Company: Research Funding. Kutlar:Celgene: Research Funding; Novartis: Research Funding; Hemaquest: Research Funding; Daiichi Sankyo Company, Ltd. and Eli Lilly and Company: Research Funding. Ataga:Adventrx: Consultancy; Daiichi Sankyo Company, Ltd. and Eli Lilly and Company: Research Funding. Zhou:Eli Lilly and Company: Employment, Equity Ownership. Heath:Eli Lilly and Company: Employment, Equity Ownership. Nwachuku:Daiichi Sankyo, Inc.: Employment, Equity Ownership. Jakubowski:Eli Lilly and Company: Employment, Equity Ownership. Winters:Eli Lilly and Company: Employment, Equity Ownership. Riesmeyer:Eli Lilly and Company: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal