Abstract
Abstract 827
We have recently shown that a consolidation therapy with bortezomib/thalidomide/dexamethasone (VTD) in multiple myeloma (MM) patients responding to autologous transplantation (ASCT) induces major tumor shrinking assessed by real time-quantitative (RQ)-PCR. Moreover we found that low levels of minimal residual disease (MRD) associated to a better progression-free survival (PFS) [GIMEMA VEL-03-096 trial, EudraCT Number 2004-000531-28: Ladetto et al, J Clin Oncol 2010]. We here present the updated results of this study at a median follow-up of 65 months. In the present analysis the following additional issues have been addressed: a) impact of MRD on PFS over time, with special interest to the role of MRD kinetics on outcome; b) impact of MRD on overall survival (OS).
Inclusion criteria and treatment schedule for this study have been already reported [Ladetto et al., J Clin Oncol 2010] and included: 1) a documented complete or very good partial remission following ASCT delivered as first line treatment; 2) no previous therapy with thalidomide or bortezomib; 3) presence of a molecular marker based on the immunoglobulin heavy chain rearrangement (IGH). MRD was assessed on bone marrow samples at diagnosis, study entry, after two VTD courses, at the end of treatment and then at six months intervals, up to clinical relapse. Patients underwent MRD detection using either qualitative nested PCR and RQ-PCR, employing IGH-derived patient specific primers as already described [Voena et al., Leukemia 1997; Ladetto et al., Biol Bone Marrow Transpl 2000]. For outcome analysis patients were grouped according to following definitions: a) MRD negativity on two consecutive samples by the most sensitive PCR method (nested PCR): full molecular remission (FMR); b) MRD negativity on two consecutive samples by RQ-PCR (less sensitive but currently better standardized, according to European Study Group on MRD detection guidelines [van der Vendel et al., Leukemia 2007]): standard molecular remission (SMR); c) post-treatment tumor load above the median by RQ-PCR: high tumor burden (HTB); d) post-treatment tumor load below the median by RQ-PCR: low tumor burden (LTB); e) recurrence of detectable MRD after FMR/SMR: molecular relapse (M-rel); f) increase of MRD levels of at least one log: active disease (AD).
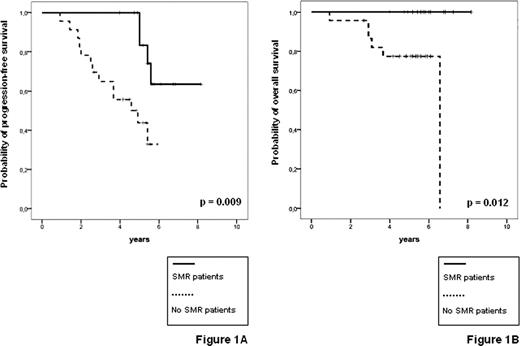
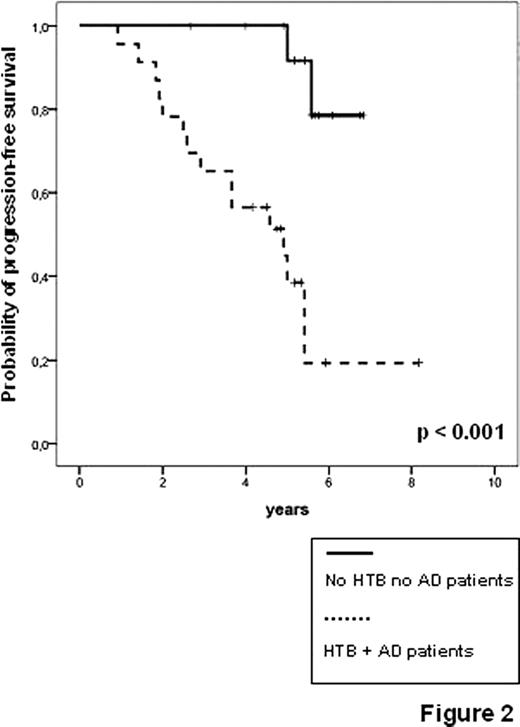
Feasibility, toxicity and clinical outcome of the trial have been already reported [Ladetto et al., J Clin Oncol 2010]. Thirty-nine patients were enrolled and median clinical follow-up from start of first line treatment is 65 months. 270 of the planned samples for MRD monitoring (86%) were actually received by the centralized lab. So far 17 relapses and six deaths have been reported. Following VTD consolidation, 7/38 evaluable patients achieved FMR (18%) and 15/38 achieved SMR (39%). Three M-rel were observed, two of them followed by clinical relapse within six months. Achievement of SMR proved highly predictive for PFS (5-years (y) PFS 82% vs 44%, p=0.009, figure 1A), as well as the presence of HTB and AD (5-y PFS 35% vs 87%, p<0.001, figure 2). Interestingly, patients with LTB and no evidence of M-rel or AD had an excellent outcome with a 5-y PFS of 87%, (even considering that molecular follow-up was incomplete due to lack of samples in the two events observed in the low risk group, figure 2). Most notably, none of the patients achieving FMR or SMR has so far died and both SMR and AD proved to be significant predictors for OS (respectively, 5y-OS 100% vs 74%, p=0.012, figure 1B, and 5y-OS 86% vs 100%, p=0.037, data not shown).
PFS (A) and OS (B) by RQ-PCR status at the end of VTD consolidation (38 evaluable patients)
PFS (A) and OS (B) by RQ-PCR status at the end of VTD consolidation (38 evaluable patients)
Our long-term results indicate that: 1) the achievement of SMR following VTD consolidation in MM patients is associated with a better outcome in terms of PFS and OS; 2) a dynamic increase in molecular tumor burden (AD), detectable by RQ-PCR, predicts late disease relapses several months before clinical recurrence. Taken together these results suggest the importance of developing tailored treatment for patients with high residual burden or showing increasing levels of MRD during follow-up, as already pursued for example in mantle cell lymphoma [Andersen et al., J Clin Oncol 2009].
Ladetto:Celgene: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Bayer: Honoraria; Mundipharma: Honoraria; Janssen-Cilag: Research Funding; Italfarmaco: Research Funding. Cavallo:celgene: Honoraria. Guglielmelli:celgene: Honoraria; Janssen-Cilag: Honoraria. Boccadoro:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen-Cilag: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Palumbo:Merck: Honoraria; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal