Abstract
Abstract 818
Graft-versus-host disease (GvHD) is a complication that results from minor and/or major MHC incompatibilities between the donor and the recipient after hematopoietic stem cell transplantation (HSCT). Although the importance of donor T effector cells (Teffs) in inducing GvHD is well-established, the early events by which host antigen presenting cells (APCs) activate allogeneic T cells are not well described. In pathogen specific immunity, after T cells migrate to the lymph node, they initially form brief contacts with dendritic cells (DCs). This phase lasts for 6–8 hours, which is termed random-walk. Following this phase, T cells establish long-lasting arrest on DCs. At least 6 hours of stable T cell-DC interaction are required for na•ve T cells to undergo clonal expansion. After the phase of long-lasting contact, T cells start to proliferate and expand. At the same time (20∼24 hrs), their interactions with DCs become very short again. It is not clear if allogeneic T cells follow a similar tri-phasic activation process. In addition, studies on autoimmunity have shown that Tregs can prevent T cell activation by prolonged interaction with APCs. However, the mechanisms by which donor regulatory T cells (Tregs) regulate T effector responses in GvHD in lymphoid tissue has not described previously.
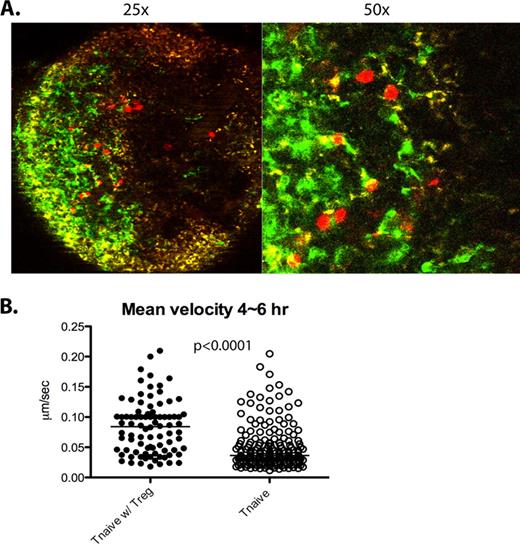
We have also observed that although the speed of donor Treg movement is very similar to na•ve T cell 4∼6 hours after BMT, the velocity of donor Tregs is much slower than the velocity of Teffs 18 hours post BMT. This slow speed persists even at 24 hours post BMT, indicating that Tregs form very stable contacts with host DCs. Interestingly, donor na•ve T cells move significantly faster when transplanted with Tregs at a 2:1 (T:Treg) ratio (Figure 1B), suggesting that Tregs interfere with interactions between na•ve T cells and DCs.
We conclude that allogeneic na•ve T cells do not go through a random-walk phase in order to interact with host DCs early post HSCT. The transplantation of Tregs with donor T cells and bone marrow cells significantly increased the velocity of donor na•ve T cells, probably diminishing the interaction of Teff cells with DCs after BMT. We believe that the large number of APCs able to present antigen in the allogeneic setting allows for rapid activation of donor Teff cells and precludes the necessity of the random-walk phase characteristic of pathogen-specific T cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal