Abstract
Abstract 777
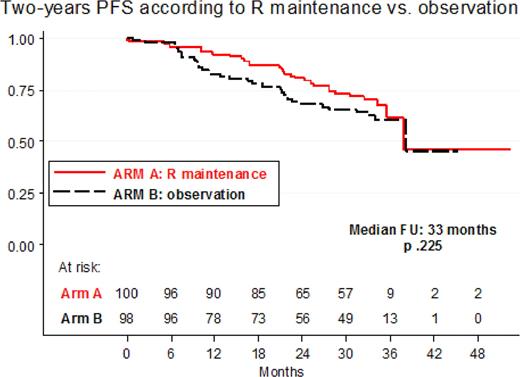
Introduction. Fludarabine containing regimens are effective in FL; the role of Rituximab (R) maintenance has been established recently. Aim of the study was to investigate efficacy and safety of R-maintenance vs. observation in elderly FL patients who respond to a brief chemo-immunotherapy with four courses of R-FND + four doses of Rituximab as consolidation. Methods. From January 2004 to December 2007, elderly patients (age 60–75) with untreated advanced stage FL who required treatment, were enrolled by 33 FIL centers. Treatment plan was: four courses of R-FND (standard doses of Rituximab, Fludarabine, Mitoxantrone, Dexamethasone) every 28 days followed by four weekly Rituximab infusions as consolidation; responding (Complete Response, CR + CRu + Partial Response, PR) patients were randomized between a short Rituximab maintenance with a single dose every two months for a total of four doses (Arm A) or observation (Arm B). Qualitative and quantitative PCR monitoring for IgH/Bcl-2 rearrangement on bone marrow (BM) was performed at diagnosis, after R-FND and R consolidation and during maintenance/observation. Results. 242 patients were enrolled at diagnosis in the study and 234 were eligible for treatment: median age was 66 yrs; advanced stage II 14%, stage III 21% and stage IV 65%; BM involvement and B symptoms were documented in 55% and 18% respectively. According to FLIPI risk patients were: Low 11%, Intermediate 34%, High 55%. According to Comprehensive Geriatric Assessment (CGA), concomitant illness were: one in 38% and greater than or equal to two in 23% of patients. Qualitative PCR analysis for IgH/Bcl-2, performed in 223 patients at diagnosis, was positive in 49%. Two hundred and two (86%) patients completed the induction treatment and were randomized between maintenance or observation; 32 were not because of stable/progressive disease (15), adverse events (nine) or other causes (eight). At the end of chemoimmunoterapy and consolidation overall response rate was 86% with 68% CR and 18% PR. After four courses of chemo-immunotherapy, 90 patients were in PR of whom 37 (41%) were converted to CR with the addition of Rituximab consolidation. As regard to Bcl2 analysis, PCR negativity was detected in 61% after R-FND and increased to 73% after R consolidation. With a median follow-up of 33 months, two-years Overall Survival and Progression Free Survival (PFS) were 93% (95% CI 92%–97%) and 77% (95% CI 71%–93%), respectively. Two-years PFS according to maintenance/observation phase was: 80% vs 68% (p.225), Figure 1. Two-years PFS rates according to FLIPI score were 87% for low/intermediate risk and 70% for high risk (p < 0.0001). A total of 1119 courses were delivered; the most frequent CTC grade 3–4 toxicity was neutropenia in 25% of the courses, with only 13 events of serious infections. Two toxic deaths during treatment (0.8%) occurred: 1 HBV reactivation and 1 Steven Johnson syndrome. In the maintenance/observation phase the following severe (WHO grade 3–4) toxicities were recorded: 15 patients experienced neutropenia, 8 cardiac events, 4 infections; no other relevant toxicities were recorded. Second malignancy were recorded in 11 patients. Conclusions. a short term chemo-immunotherapy R-FND + Rituximab consolidation is able to achieve high CR rate and a good two-years PFS in elderly FL patients. Good results were also observed in higk-risk FLIPI score. Two-years PFS of 80% in patients randomized to R maintenance was promising although there was no statistical difference compared to observation. This finding may be attributed to the relatively short follow-up or to the choice of a short course of Rituximab maintenance (only four doses) or both.
Figure 1
Disclosures:
Vitolo:Jannsen-Cilag: Speakers Bureau; Celgene: Speakers Bureau; Roche Italy: Speakers Bureau. Off Label Use: The study includes use of Rituximab as maintenance in responding patients after first line chemoimmunotherapy. Gamba:Roche Italia: Employment. Supekar:Roche Italia: Employment.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal