Abstract
Abstract 776
Waldenstrom's macroglobulinemia (WM) and related-disorders (Marginal Zone Lymphoma: MZL, and non immunoglobulin IgM lymphoplasmacytic lymphoma: LPL) are rare diseases Very few randomized trials were reported in this setting. Most commonly patients with WM are initially treated with an alkylating agent, such as chlorambucil (CBL) or with a nucleoside analogue such as fludarabine (F) or 2CdA, alone or in association with monoclonal antibody.
WM1 study was a prospective international randomized open-label study that included patients with previously untreated WM MZL and LPL. At registration, patients were stratified as having WM, SLVL, or LPL, and were randomized in the two arms. The aim of the study was to compare the efficacy of oral CBL at a dose of 8 mg/m2 for 10 days every 28 days to a maximum of 12 cycles with oral F at a dose of 40 mg/m2 orally for 5 days every 28 days to a maximum of 6 cycles. 418 patients were enrolled into the study from 07/01 to12/09. 414 patients were included and 405 received at least one course of chemotherapy. There were 339 WM, 37 MZL and 38 LPL with a median age of 68 years (40-89). 207 patients were randomized in the F arm and 207 patients in the CBL arm. At inclusion, the median of haemoglobin (g/l), platelets (Giga/l), albumin (g/l) and beta 2 microglobulin (mg/l) were 9.9, 218, 37.1 and 3.47 respectively.
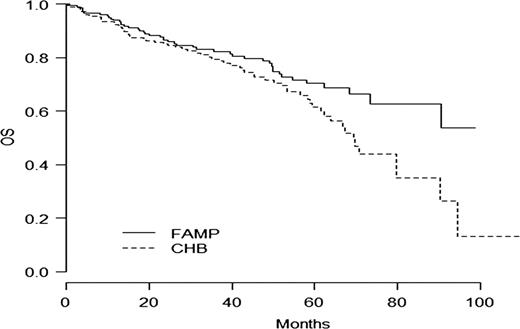
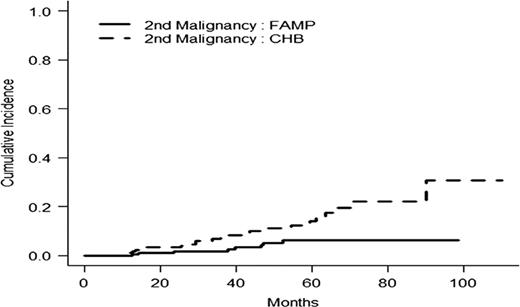
In intention to treat basis, the overall response rate (CR+PR) was 47.8 % in the F arm versus 38.6% in the CBL arm (p=0.06). With a median follow-up time of 35.9 months, the median of progression free survival time (PFS) and disease free survival (DFS) were statistically longer in the F arm: PFS 36.3m vs 27.1 m ( p=0.01) and DFS 38.3m vs 19. 9m (p= 0.0006). In WM group, factors influencing negatively PFS were CBL arm, albumin< 35g/l, platelets<100 G/l and age> 70 years. Main toxicity was haematological with 17/203 (8.3%) vs 18/203 (9%) of grade III- IV thrombocytopenia and 50/203 (24.6%) vs 39/202 (19.3%) of grade III-IV anemia in F and CBL arms respectively. Overall survival rate at 5 years was 61.4% [52.9;71.3] in CBL arm and 70.3% [62.7-78.8] in F arm (p=0.04) (Fig 1). Cumulative Incidence of second malignancies (solid tumors and haematological malignancies except Richter syndrome (RS)) was statistically higher in the chlorambucil arm (25 versus 8, p= 0.004) (Fig 2). The number of RS was 8 in F arm and 9 in CBL arm.
Cumulative incidence of second malignancies in randomized group (p=0.004)
F by oral route is a safe and effective ambulatory treatment in WM and close related disorders patients, even in the elderly and more effective than CBL with a duration of response over 3 years. An unexpected finding was a statistically higher number of second malignancies in the C arm and we cannot rule out an oncogenic role of CBL in this setting. Of note, we stress that it is the first time that a front- line treatment has a significant impact on overall survival in WM patients.
Leblond:mundipharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; genzyme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Tournilhac:Amgen: Research Funding; Mundipharma: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal