Abstract
Abstract 731
Paroxysmal Nocturnal Hemoglobinuria (PNH) is an acquired disorder of hemopoietic stem cells (HSCs). Affected individuals experience intravascular hemolysis and have a predisposition to thromboembolism, renal impairment and pulmonary hypertension. These symptoms vary in severity, but in general, the higher the proportion of PNH blood cells produced, the more severe the symptoms. The molecular pathogenesis in PNH is known to relate to a single gene mutation in the X-linked phosphatidylinositol glycan class A gene (PIG-A) which causes a complete or partial deficiency of glycophosphatidylinositol (GPI) anchored proteins leading to the symptoms of the disease. The recent development of eculizumab therapy in PNH has had a dramatic impact in reducing both morbidity and mortality in the disease but PNH remains incurable. A sub-optimal response to eculizumab, certainly when assessed by transfusion requirements, is often due to the underlying bone marrow failure that is considered to be universally present in PNH. The factors that lead from the development of a mutant clone to clonal expansion and symptomatic disease are poorly understood but are the key to improving responses and potentially to move towards a cure.
Bone marrow failure appears to provide the environment necessary for expansion of PNH clones and small PNH clones are often detected in aplastic anemia and less commonly in myelodysplasia. There is no evidence that PNH HSCs have an intrinsic proliferative advantage compared to normal HSCs and an immune-mediated extrinsic suppression of normal hematopoiesis with a selective advantage for the PNH cells over residual normal stem cells is likely to explain the preferential development of PNH clones concurrent with bone marrow failure.
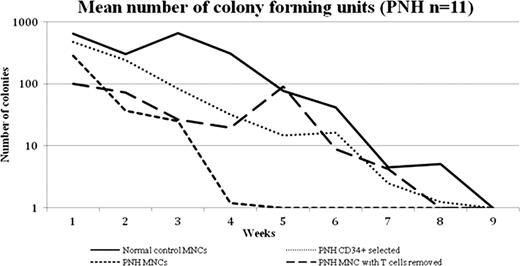
To gain a better understanding of clonal expansion in PNH we have developed an in vitro PNH bone marrow culture model using a stromal cell line which allows PNH stem cells to be maintained in long term cultures and their capacity to form progenitor cells in myeloid colony forming assays to be assessed. We have evaluated bone marrow from 11 patients with PNH (median age 47 years, median granulocyte clone size 95.3%) and 10 normal controls (median age 42 years) within these long term bone marrow culture experiments. Unmanipulated bone marrow mononuclear cells (MNCs), CD34 selected cells and MNCs with their T-cell component depleted were used in this model. This in vitro model provides the environment for PNH stem cells to be maintained for up to eight weeks and, unlike previous studies, produce progenitor cells. When the patient's MNC's are used to seed the culture system there is poor growth of the culture which is only maintained for a median of 2 weeks. However if CD34 selected cells are used then the cultures are maintained for up to 8 weeks (similar to the normal controls) suggesting that there is a component within the MNC's that is responsible for suppressing the marrow culture which is removed by CD34 select. We next selectively removed the T-cells from the PNH MNC's and demonstrated that the marrow cultures now survived to the extent of the controls (see Figure). This demonstrates that the immune suppression in PNH resides in the T-cells. The progenitor cells produced in both the CD34 selected or the T-cell depleted MNCs over the course of the long term culture experiments show an increase in the proportion of normal progenitors the longer the cultures are maintained. This supports the hypothesis that PNH stem cells have no intrinsic proliferative advantage over normal HSCs and that T-cells are the critical cell suppressing the normal hematopoiesis in PNH. We are now examining the specific cell type that causes the myelosuppression in PNH and which will facilitate a targeted approach to the treatment of bone marrow failure in PNH and related disorders.
Kelly:Alexion Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Richards:Alexion Pharmaceuticals: Honoraria, Speakers Bureau. Arnold:Alexion Pharmaceuticals: Honoraria. Hill:Alexion Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Hillmen:Alexion Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal