Abstract
Abstract 720
Platelets are currently limited to 5 days of storage at room temperature to prevent growth of bacteria to high levels. Cold storage of platelets could reduce bacterial proliferation but platelets stored in cold for over 48 hours are cleared rapidly from circulation through the hepatocyte Ashwell-Morell (AM) receptor thus limiting the applicability of cold temperatures to platelet storage. We used a temperature cycling method to store human platelets in the cold without decreasing their in vivo recovery in an immunodeficient (SCID) animal model of transfusion.
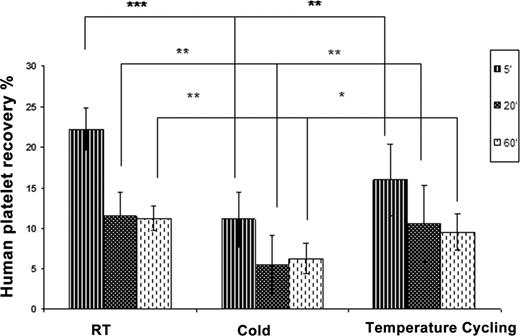
Temperature cycled (TC) apheresis human platelets were stored in the cold (4°C) for 12 hours and then incubated at 37°C for 30 minutes before returning back to cold storage. The TC (37°C pulses for 30 minutes at 12 hour intervals) was continued for 2, 5 and 7 days. Human platelets stored either at room temperature (RT), cold or TC for 2, 5 and 7 days were infused into 6 to 8 SCID mice per group and their in vivo recovery in circulation was determined at 5, 20 and 60 minutes after transfusion by flow cytometry. Carbohydrate exposure on the surface of the platelets was analyzed for galactose by Erythrina cristagalli agglutinin (ECA), and for β-GlnNAc by succinyl wheat germ agglutinin (sWGA) using flow cytometry. Involvement of the AM receptor was examined by monitoring clearance of cold stored platelets in the presence of asialofetuin, a competitive ligand for the receptor.
Human Platelet Recovery (% of total platelets circulating) * p< 0.05, ** p< 0.01, *** p< 0.001
Human Platelet Recovery (% of total platelets circulating) * p< 0.05, ** p< 0.01, *** p< 0.001
Binding of the galactose specific lectin, ECA, was increased by 142±22% from RT to cold platelets (P<0.01) as previously reported. However, binding of ECA was also increased by 134±16% from RT to TC platelets (P<0.01). β-GlnNAc exposure, as measured by sWGA lectin binding, was increased after cold and TC storage by 222±65% (P<0.01) and 197±14% (P<0.01), respectively, when compared to RT platelets. Platelets stored in the cold for >48 hours have been reported to be cleared through the hepatic AM receptor which recognizes asialocarbohydrates. Co-injection of asialofetuin significantly improved the recovery of two-day cold stored platelets from 9.5±5.1% to 18.4±7.3% (P<0.05) and 4.8±3.7% to 12.1±4.9% (P<0.01), at 5 min and 20 min post injection, respectively. Native fetuin did not alter the clearance of cold platelets. However, there was no significant increase in the recovery of TC platelets in the presence of asialofetuin as compared to fetuin injection (P>0.28), even though the TC platelets, like cold platelets, have significantly increased β-galactose exposure.
Our results indicate that ‘temperature cycling' during cold storage of platelets may be an effective method to store human platelets up to 7 days without loss of in vivo recovery after transfusion when compared to RT platelets. Temperature cycling does not alter the cold induced increases in β-gal or β-GlcNAc expression which suggests that there are other mechanisms besides binding to the AM receptor that mediate clearance of platelets stored in the cold for >48 hours.
The findings and conclusions in this abstract have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal