Abstract
Abstract 646
Adoptively transferred virus or tumor antigen-specific T-cells have demonstrated efficacy in initial clinical trials, but while clearance of viral infection is sustained, responses to tumor-specific T-cells are usually short-lived. Therefore, current efforts are focused on distinguishing attributes of virus-specific T-cells that contribute to their persistence and formulating strategies to sustain anti-tumor effects of T-cell (TC) therapies. We have developed an in-vivo model to compare the relative efficacy of T-cells specific for a tumor antigen (WT-1) versus T-cells specific for a viral antigen (CMVpp65) using human colon carcinoma cells that express the oncofetal protein WT-1 which were transduced to co-express CMVpp65 (WT-1[+] cocapp65) as a surrogate system.
Groups of 6 NOD/Scid-IL2Rgc-KO/J mice (NSG) were each subcutaneously injected with 3 × 105 WT-1 [+] cocapp65 on the R flank. Each animal was also injected with 3 × 105 cells from a WT-1[+] ovarian carcinoma cell line (SKOV3-A2) on the L shoulder to compare the efficacy of WT-1 specific T-cells (WT1-CTLs) against different WT-1 [+] tumor cell types. Expression of WT-1 protein is lower in SKOV3-A2 than in cocapp65 cells. Both tumors are HLA A0201[+] and were transduced to express a GFP-firefly luciferase gene. T-cells were administered intravenously 5 days after tumor injection to enable vascularization and tumor growth was quantitated using bioluminescence.
These experiments evaluated (1) the relative capacity of CMVpp65 specific T-cells (CMV-CTLs) versus WT-1 CTLs to eradicate WT1[+] cocapp65 cells that co-express a viral and tumor antigen (2) the relative efficacy of WT-1 CTLs against 2 different HLA A0201 [+] WT-1 expressing tumors; an ovarian carcinoma and a colon carcinoma, and (3) the contribution of IL-15/15Rα complex in augmenting the efficacy of antigen specific T-cells by using intraperitoneally (i.p) injected Baf-3 cells transduced to express human IL-15/15Rα complex. The treatment groups were as follows: (1) Control – no T-cells + IL-2 (2000 U) (2) Control – no T-cells + IL-15/IL-15Rα (5 × 106 baf-3 cells) (3) WT1 CTLs + IL-2 (2000 U) (4) CMV-CTLs + IL-2 (2000 U) (5) WT1 CTLs + IL-15/IL-15Rα (5 × 106 baf-3 cells) (6) CMV-CTLs + IL-15/IL-15Rα (5 × 106 baf-3 cells). IL-2 and irradiated baf-3 cells were administered intraperitoneally twice weekly.
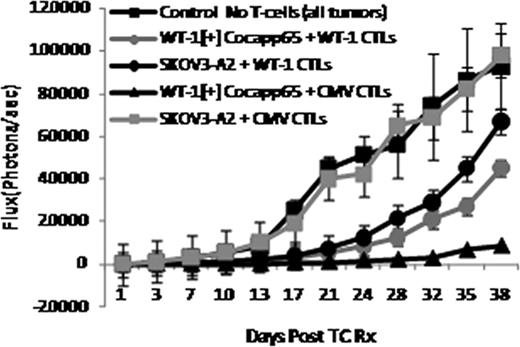
When the doses of antigen specific interferon gamma (IFNg) [+] T-cells were equivalent in the infused CMVpp65 and WT1 specific T-cells, the CMV-CTLs induced greater, and more sustained suppression of the growth of the WT-1[+] cocapp65 cells in-vivo than the WT-1 CTLs (Fig.1). The anti-tumor activity of the WT-1 CTLs was greater against WT-1[+] cocapp65 than against the WT-1[+] ovarian carcinoma (SKOV3-A2), potentially reflecting the higher expression of WT-1 in cocapp65. The SKOV3-A2 tumor began to re-grow by 24 days post T-cell infusion approaching the size of control tumors by day 38, while the WT-1[+] cocapp65 still demonstrated slower growth through day 38. The addition of IL-15/15Rα increased the efficacy of the transferred T-cells, the difference being more pronounced for the anti-tumor activity of WT-1 CTLs.
Comparative Efficacy of CMV and WT-1 CTLs against Human Tumor Targets Co-expressing CMVpp65 and WT-1
Comparative Efficacy of CMV and WT-1 CTLs against Human Tumor Targets Co-expressing CMVpp65 and WT-1
These studies demonstrate that equivalent doses of IFNg[+] WT-1 CTLs can also suppress WT-1[+] cocapp65 tumor xenografts, but are less effective than CMV-CTLs, and that IL-15 supplementation augments the cytotoxic activity of the CTLs in-vitro and enhances the duration of the anti-tumor effects in-vivo. This model permits side by side comparisons of the anti-tumor activity of human T-cells directed against viral and tumor antigens expressed on the same clonogenic human tumor target. Because both responses are directed against the same cells, this model could thereby facilitate identification of the distinguishing features of T-cells specific for viral or tumor antigens as well as differences in the presentation of viral and oncofetal “self” antigens by tumor cells that contribute to disparities in their anti-tumor activity and persistence in-vivo.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal