Abstract
Abstract 5183
The advantage of using interim 18F-fluorodeoxyglucose (FDG) positron-emission tomography (PET) scan in the clinical work-up of patients with non-Hodgkin's lymphoma (NLH) is unclear. Data from meta-analyses are inconclusive, mainly because of the low number of patients evaluated and heterogeneity among studies. New clinical investigations, focused on this topic, have been recently published. We conducted an updated systematic review on the role of 18PDG-PET for the interim evaluation in patients with aggressive lymphomas.
Medline, Embase, Scopus and Databases were searched for relevant studies through March 2011. We included studies that evaluated FDG-PET performed between the first and the fourth cycle of first-line chemotherapy. Patients were selected when they were evaluated for response assessment with interim 18FDG-PET. For each study, we constructed a 2 × 2 contingency table consisting of true positive (TP), false positive (FP), false negative (FN), and true negative (TN), where all patients were categorized according to whether they were PET positive or negative, and whether they experienced treatment failure. A meta-analysis of the prognostic accuracy was performed.
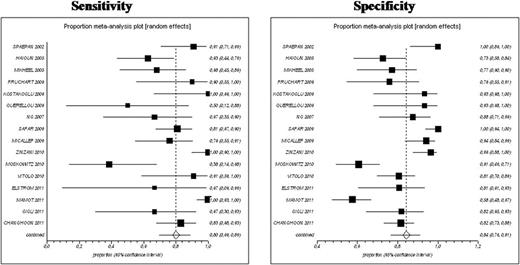
We selected 16 out of 46 studies, involving 1256 patients with aggressive NHLs (90% with Diffuse Large B-Cell Lymphoma); 1157 patients met our inclusion criteria and were considered for the final analysis. Interim18FDG-PET, performed after a median of 3 cycles of chemotherapy (range 1–6 cycles), gave true and false negative results in 641 (56 %) and 65 (6%) patients, respectively. Therefore, 18FDG-PET had an overall sensitivity of 0.80 (95% CI, 0.69 to 0.88) and a specificity of 0.84 (95% CI, 0.76 to 0.90) (figure). Heterogeneity among studies was substantial.
Because of data heterogeneity, interim 18FDG-PET for aggressive NHL patients remains an unproven test for routine clinical practice. Its role should be further evaluated in clinical researches with homogeneous treatments and standardized imaging.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal