Abstract
Abstract 5055
Myelodysplastic syndrome (MDS) represents a heterogenous disorder characterized by ineffective hematopoiesis and propensity to evolution to acute myelogenous leukemia (AML). Azanucleosides like 5-azacitidine (AZA) and decitabine-5-aza-2'-deoxycytidine (DAC) improve survival (Fenaux, 2008; Silverman, 2002 and Steensma, 2009). In this retrospective study, we aimed to evaluate: (1) clinical outcomes in Veterans (pts) diagnosed with MDS and treated with either 5-AZA or DAC and (2) survival outcomes according to number of cycles received of either hypomethylating agent.
After obtaining IRB approval, data was collected from the Michael E. DeBakey VA Medical Center cancer registry and pharmacy records between January 1, 2000 and June 1, 2011. For analysis, pts were included in the study if they were ≥18 years (y), had morphological evidence of MDS per bone marrow examination at the time of diagnosis and were treated with either hypomethylating agent (but not both) as first-line therapy for 2 cycles or more. Pts with de-novo AML prior to treatment were excluded. Pts received AZA at 75 mg/m2/day (d) (dose range, 50–75 mg/m2/d) (d 1–7) or DAC at 20 mg/m2/d (dose range, 15–20 mg/m2/d) (d1-5) at four weeks interval. The primary end-point was Overall Response Rate (ORR) which included Complete Response (CR), Partial Response (PR) and Hematological Improvement (HI) according to IWG 2006. Secondary end-point was Overall Survival (OS) per treatment group.
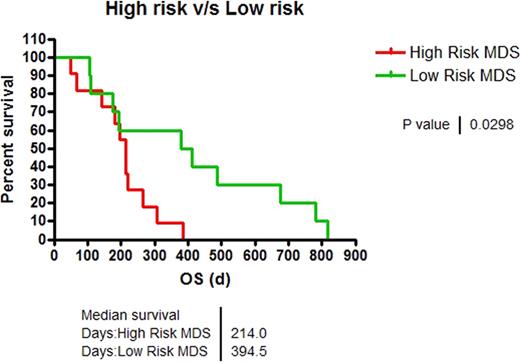
We identified 39 pts who underwent treatment with azanucleosides. 18 pts were excluded either because of de-novo AML diagnosis at presentation, they had received < 2 cycles of treatment, or had received > 2 cycles of both agents, 11, 5, and 2 pts, respectively. Demographics are depicted in table 1. 21 pts were available for analysis, from which 2/21 (10%) and 19/21 (90%) were female and male, respectively. Median age was 66 y (range, 55–78 y) and 63 y (range, 56–78 y) for pts treated with AZA vs. DAC (P=0.458). 9/21 (43%), 5/21 (24%), 7/21 (33%) pts had good, intermediate and poor-risk karyotypes. 13/21 (62%) and 8/21 (38%) pts received AZA and DAC, respectively. ORR was 15% vs. 38%, for AZA vs. DAC (P=0.618). Per IPSS score, 52% and 48 % of the pts were high-risk (high and Int-2) and low-risk (Int-1 and low), respectively. ORR was 30% vs. 18%, for low-risk vs. high-risk pts (P=0.635). OS was superior in low-risk compared to high-risk pts (394d vs. 214d, P=0.029, Figure 1). Pts who responded to therapy (CR+ PR +HI), had superior OS compared to pts with stable or progressive disease (411d vs. 194d, P= 0.01). There was no statistical difference in OS between different age groups. OS in pts receiving ≥4 or <4 cycles of treatment was 486d vs. 187d for AZA-treated pts (P=0.005) and 411d vs. 174d for DAC group (P=0.04). Leukemia transformation was seen in 5 vs. 2 pts treated with AZA and DAC. For this subset, Time to Leukemia transformation (TTL) was 102d vs. DAC 272d (P=0.65).
Patient Characteristics
| . | Azacitidine Tx N=13 . | Decitabine Tx N=8 . |
|---|---|---|
| Demographics Median age (years) | 66 | 63 |
| Male:Female | 12:1 | 7:1 |
| White:Black | 11:2 | 7:1 |
| IPSS (prior to Tx) | ||
| Low | 1 | 0 |
| INT-1 | 4 | 5 |
| INT-2 | 5 | 2 |
| High | 3 | 1 |
| Median Number of Cycles | 3 | 3 |
| Response CR+PR+HI | 2 | 3 |
| SD | 6 | 4 |
| PD | 5 | 1 |
| Hematologic Improvement HI-E | 2 | 3 |
| HI-P | 1 | 1 |
| HI-N | 0 | 1 |
| No. of Patients Progressed to AML | 5 | 2 |
| Median Time of Progression to AML (from time of treatment to diagnosis of AML) (Days) | 102 | 271 |
| Median Overall Survival (from time of treatment to death of any cause) (Days) | 215 | 261 |
| . | Azacitidine Tx N=13 . | Decitabine Tx N=8 . |
|---|---|---|
| Demographics Median age (years) | 66 | 63 |
| Male:Female | 12:1 | 7:1 |
| White:Black | 11:2 | 7:1 |
| IPSS (prior to Tx) | ||
| Low | 1 | 0 |
| INT-1 | 4 | 5 |
| INT-2 | 5 | 2 |
| High | 3 | 1 |
| Median Number of Cycles | 3 | 3 |
| Response CR+PR+HI | 2 | 3 |
| SD | 6 | 4 |
| PD | 5 | 1 |
| Hematologic Improvement HI-E | 2 | 3 |
| HI-P | 1 | 1 |
| HI-N | 0 | 1 |
| No. of Patients Progressed to AML | 5 | 2 |
| Median Time of Progression to AML (from time of treatment to diagnosis of AML) (Days) | 102 | 271 |
| Median Overall Survival (from time of treatment to death of any cause) (Days) | 215 | 261 |
OS of High Risk vs. Low Risk pts.
In our study, therapy with AZA and DAC was associated with ORR comparable to historical data of about 25%. OS was superior in pts receiving ≥4 cycles of treatment of either regimen. Despite the small number of pts and retrospective nature of our study, our data suggest that TTL is similar for both agents. Prospective studies, in veteran pts, evaluating efficacy of AZA vs. DAC are needed.
OS in pts receiving <4 or ≥4 cycles of treatment.
Off Label Use: Use of azacytidine and decitabine for low risk MDS. Yellapragada:Bristol Myers Sqibb: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal