Abstract
Abstract 4894
Pretreatment karyotypes of leukemic cells provide the core for risk-stratification schemes in acute myeloid leukemia (AML) and cytogenetic abnormalities are well established as the strongest prognostic factor for response to therapy and for survival in AML patients. Several cytogenetic risk classifications have been built to stratify AML patients. Only the recent European Leukemia Net (ELN) recommendations by an international panel proposed a new standardized cytogenetic reporting system for AML. More importantly, few studies attempted so far to validate the ELN requirements for risk assessment in AML patients treated with allogeneic hematopoietic stem cell transplantation (HSCT). We investigated whether our series of adult AML patients treated with HSCT could validate the ELN classification criteria. We also assessed the prognostic value of this new risk stratification scheme.
ELN cytogenetic reporting criteria were applied to a series of 110 adult AML patients from a single institution in the Middle East for whom complete cytogenetic data from pretreatment leukemic marrow were available and patients were assigned to the proposed cytogenetic risk subgroups, accordingly. We compared outcome for the different prognostic subgroups. Parameters analyzed were overall survival (OS) and event-free survival (EFS). Our AML group included 62 (56%) patients treated in their first remission (CR1), while most non-CR1 AML patients were treated with HSCT in CR2. The median age of all patients was 25 years (range: 14–57).
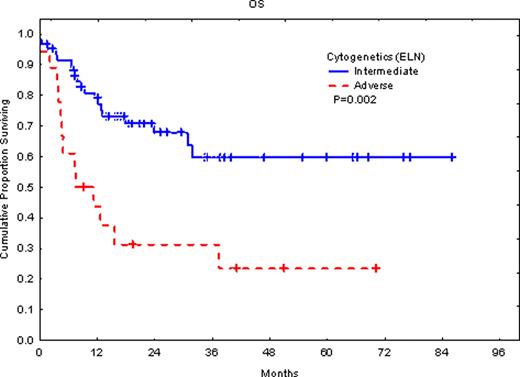
The patient group included 32 (29%) patients with favorable risk, 60 (55%) in the intermediate-risk group, and 18 (16%) in the adverse-risk group. When all the three groups were considered, there was significant difference in overall survival (OS) (P=0.007) and event-free survival (EFS) (P=0.007). However, there was no significant difference between our favorable-risk group and the intermediate-risk group. This is most likely due to selection bias in the favorable group (e.g. CD56 expression and other unfavorable markers). The adverse-risk group had significantly (P=0.002) shorter survival with median survival of 7.5 months vs. the intermediate-risk group where median survival has not been reached with an average follow-up of 46 months. When we attempted to consider only patients treated with HSCT in the first remission, there were too few patients in the intermediate-risk group (7 patients) for statistical evaluation.
The ELN criteria proofed valuable in risk-stratifying our set of adult AML patients who were treated with HSCT and represent a Middle Eastern population. Our series clearly validated the ELN classification. The ELN classification needs further validation in patients treated with HSCT in first remission.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal