Abstract
Abstract 4627
The internal tandem duplication (ITD) and tyrosin kinase domain (TKD) D835 mutation in the Fms-like tyrosine kinase receptor-3 gene (FLT3) are perhaps the most studied prognostic molecular markers in acute myeloid leukemia (AML). However most of the studies have been in patients treated with standard intensive chemotherapy. The prognostic significance of FLT3, especially the TKD D835, in AML patients treated with intensive chemotherapy followed by allogeneic hematopoietic stem cell transplantation (HSCT) is not well studied.We studied the prognostic value of both FLT3-ITD and FLT3-D535 mutations in patients with AML treated with HSCT.
Samples from 87 patients with AML were analyzed for FLT3 ITD and D835 mutations using fragment length analysis for ITD and restriction enzyme digestion followed by fragment length analysis for D835. All patients were diagnosed with AML, treated with intensive chemotherapy, then HSCT. They included 66 (76%) patients with intermediate cytogenetic abnormalities and 21 (24%) patients with adverse cytogenetics.
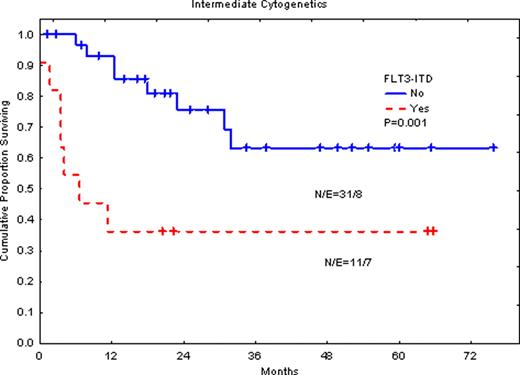
ITD mutation was detected in 18 patients (21%) patients, while the D835 mutation detected in 6 patients (7%). FLT3-ITD was associated with significantly shorter overall survival (OS) when all patients are considered (P=0.006). When only patients with intermediate cytogenetic abnormalities treated with HSCT in their first complete response (CR1) are considered, FLT3-ITD was significantly associated with poor OS (P=0.001). Event free survival (EFS) was also significantly shorter in patients with the FLT3-ITD (P=0.005). Similarly, D835 mutation was associated with significantly shorter OS (P=0.002) in this group of patients. Surprisingly, in patients with poor cytogenetic abnormalities, patients with FLT3-ITD had significantly longer survival (P=0.05), although the number is too small.
Our data suggests that FLT3 mutations, both ITD and D835, remain poor prognostic markers in patients with AML treated with intensive chemotherapy followed by HSCT, but only in patients with intermediate risk cytogenetic abnormalities and this may not be relevant in patients with high-risk AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal