Abstract
Abstract 444
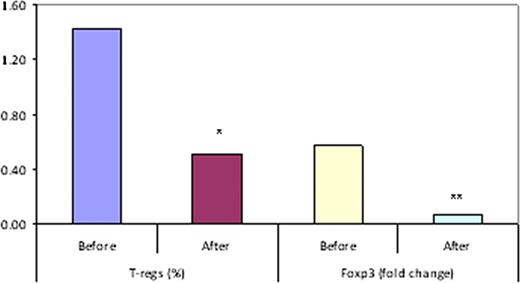
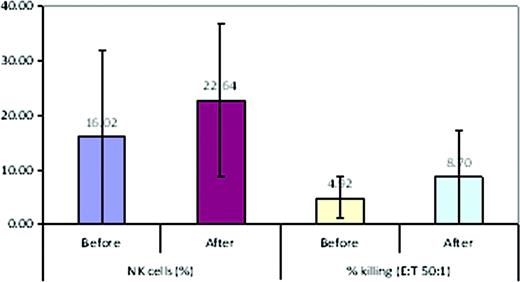
The CC chemokine receptor 4 (CCR4) is expressed on malignant T-cells in cutaneous T-cell lymphoma (CTCL) and also on the surface of regulatory T cells (T-regs). T-regs also express the transcription factor, foxp3 and suppress effector immune cells, including natural killer (NK) cells. Thus, increased T-regs in the tumor microenvironment is associated with impaired anti-tumor immunity. KW-0761, a defucosylated, humanized monoclonal antibody, binds to CCR4 and induces effective antibody-dependent cellular cytotoxicity (ADCC) against CCR4+ malignant T-cells. The phase I/II clinical trial to determine the safety and efficacy of anti-CCR4 antibody (KW-0761) included a translational component to evaluate its effects on T-regs and NK cells in CTCL patients.
Peripheral blood mononuclear cells (PBMCs) were collected from 20 patients [10 with mycosis fungoides (MF) and 10 with Sézary syndrome(SS)] pre- and post-treatment at two centers for flow cytometry analysis of CD3+CD4+CD25+CD127- T-regs, CCR4+ T-regs, and CD3-CD56+CD16+ NK cell subsets. Total RNA was extracted from PBMCs, and foxp3 and CCR4 mRNA were quantified using real-time PCR. The standard 4-hour 51Cr release assay was used to assess the cytotoxicity of NK cells.
Our results suggest that in addition to ADCC towards malignant T-cells, the anti-neoplastic activity of the anti-CCR4 antibody (KW-0761) may include a reduction in T-regs in most CTCL patients and a subsequent increase in NK numbers and function in some patients. Follow up studies need to be performed to confirm these findings.
Ni:KYOWA HAKKO KIRIN CO., LTD: Research Funding. Kim:kyowa: Consultancy, Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Allos: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millenium: Consultancy. Duvic:KYOWA HAKKO KIRIN CO., LTD: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal