Abstract
Abstract 4404
Benzene, widely used in the chemical industry, is known to be myelotoxic, and to cause acute myeloid leukemia in humans. Although the mechanism has not been elucidated, benzene toxicity is considered to occur only after metabolic activation. Myeloperoxidase (MPO) is synthesized and secreted by myeloid cells catalyzes the formation of hypochlorous acid (HOCl), a powerful oxidant derived from chloride ions and hydrogen peroxide (H2O2). HOCl plays key roles in host defense by oxidizing the cellular constituents of invading pathogens. At the same time, HOCl is also capable of damaging proteins and nucleic acids in host tissue. By damaging the DNA of host cells, MPO-induced DNA halogenation (halogenative stress) might contribute to the carcinogenesis.
Because the findings of in vivo and in vitro research so strongly implicate the involvement of reactive oxygen species (ROS) in benzene-induced toxicity, focusing on 1,2,4-benzenetriol (BT), a benzene metabolite that generates ROS by autoxidation, we designed a study to investigate the toxicity of benzene leading to leukemogenesis, specifically examining the cytotoxic effects of BT on a human myeloid cell line, a class of cells from the organ mainly affected by benzene.
After exposing human myeloid cell line HL-60 to BT, we investigated the cellular effects, including apoptosis, ROS generation, DNA damage (halogenated-DNA and 8-oxo-deoxyguanosine (8-oxo-dG)), and protein damage (halogenated tyrosine). We also investigated how the cellular effects of BT were modified by H2O2 scavenger catalase, HOCl scavenger methionine, and 4-aminobenzoic acid hydrazide (ABAH), a myeloperoxidase-specific-inhibitor. Apoptosis was measured by Annexin V-fluorescein isothiocyanate and propidium iodide double-labeling kits. Intracellular ROS were determined flow-cytometrically using ROS-sensitive fluorescent probes. Halogenated-DNA was measured by flow cytometry after immunostaining, using a novel monoclonal antibody (mAb2D3) which recognizes the HOCl-modified 2’-deoxycytidine residue, 5-chloro-2’-deoxycytidine. 8-oxo-dG was measured by electrochemical detection after HPLC separation and halogenated tyrosines were detected immunocytochemically.
BT increased the levels of apoptosis and of ROS including superoxide (O2•−), H2O2, HOCl, and the hydroxyl radical (•OH). Catalase, ABAH, and methionine each inhibited the increased apoptosis caused by BT; and catalase and ABAH inhibited increases in HOCl and •OH. Indicating that HOCl was generated via the H2O2–MPO–HOCl system, this inhibition of HOCl generation by both catalase and ABAH demonstrates that, in HL-60 cell samples exposed to BT, H2O2 was certainly metabolized to HOCl by MPO. It also suggests that HOCl might trigger BT-induced apoptosis of HL-60 cells. We then investigated whether this cytotoxicity of BT was related to the induction of DNA damage. We evaluated the halogenation of DNA by HOCl, and 8-oxo-dG induction by •OH. While BT exposure increased halogenated DNA, this increase was inhibited by catalase, methionine, and ABAH. BT exposure did not, however, increase 8-oxo-dG. To confirm the induction of halogenative stress by BT, we investigated HOCl-induced protein damage in the form of halogenated tyrosines. BT exposure also increased the amount of halogenated tyrosines.
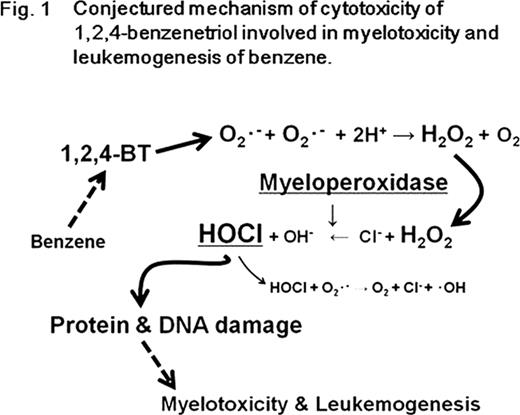
In this study we have constructed a novel hypothesis (Figure 1): exposure to BT increases O2•− generation, possibly by autoxidation; this O2•− is chemically or enzymatically converted to H2O2; this H2O2 is metabolized to HOCl by MPO; whereupon this HOCl halogenates DNA and proteins, thus inducing myelotoxicity or leukemogenesis. The high expression of MPO from myeloid cells, along with the fact that halogenated DNA can cause gene mutation and epigenetic changes, may explain how benzene is involved in bone marrow disorders or myeloid leukemia. Previous studies of benzene toxicity have reported that MPO plays a role in the bioactivation of benzene’s phenolic metabolites. Here, we have shown for the first time that a benzene metabolite, BT, is capable of generating HOCl and consequent halogenative damage via the H2O2–MPO–HOCl system. Our findings lend strong support to the conjecture that BT-induced DNA halogenation is a primary reaction in the leukemogenesis associated with benzene.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal