Abstract
Abstract 4389
Autologous hematopoietic stem cell transplantation (ASCT) has become an integral part of the treatment for multiple myeloma (MM). In addition, it is standard practice to collect enough stem cells for more than one transplant. Therefore, it is critical to have an effective mobilization strategy in order to efficiently collect sufficient numbers of CD34+ cells. Administering granulocyte-colony stimulating factor (G-CSF) alone to MM patients can produce sufficient CD34+ yields in the majority of patients. However, some patients may require > 4 apheresis days to achieve those yields. In addition, some patients may fail to collect enough CD34+ cells for ASCT. Plerixafor was approved in 2008 to be used in combination with G-CSF to mobilize hematopoietic stem cells to the peripheral blood. Plerixafor can increase the average daily CD34+ yields by 3-fold. However, since the majority of patients can collect with G-CSF alone, a plerixafor algorithm was developed in 2009 to judiciously administer plerixafor only to those patients at higher perceived risk for mobilization failure. Administration of plerixafor is based on a peripheral blood CD34+ count drawn after 3 days of G-CSF and subsequent CD34+ collection yields.
G-CSF 10mcg/kg/day (given daily or divided into twice daily) was administered subcutaneously from day 1 to 4. On day 4, a peripheral absolute CD34+ cell count was drawn. If the absolute CD34+ cell count was ≥ 12 cells/mm3 then apheresis started on day 5. If the absolute CD34+ cell count on day 4 was < 12 cells/mm3 plerixafor 240mcg/kg was administered subcutaneously the evening prior to apheresis beginning on day 5. During apheresis, if the CD34+ yield was < 1.0×106 CD34+/kg or 50% less than the previous collection, plerixafor was initiated. The minimum collection yield for all patients was 4.0×106 CD34+/kg. The maximum number of apheresis days was 5. Previous therapy was also examined.
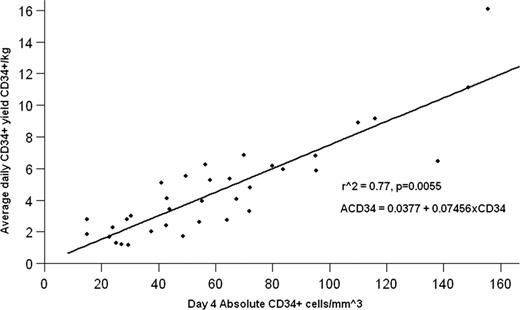
From October 2009 to May 2011, 68 multiple myeloma patients were mobilized with G-CSF +/− plerixafor. Ninety-three percent (63/68) of patients achieved the minimum collection yield of 4.0×106 CD34+/kg. Ninety-nine percent (67/68) of patients achieved a yield of at least 2.0×106 CD34+/kg. Forty-four percent (30/68) of the patients required at least 1 dose of plerixafor with the majority requiring it prior to the first apheresis (83%). The median days of apheresis was 2 (range 1–5). The overall average yield on the first apheresis day was 4.35×106 CD34+/kg (95% CI +/− 0.64). The overall average total yield was 8.71×106 CD34+/kg (95% CI +/− 0.93). Sixty percent (41/68) and 76% (52/68) of patients collected ≥ 6.0×106 CD34+/kg in ≤ 2 days and ≤ 4 days of apheresis, respectively. The average daily yield (ACD34) for G-CSF alone can be predicted by ACD34 = 0.0377+ 0.07456xCD34 (see figure). ACD34 after plerixafor + G-CSF can be predicted by the equation 3(0.0377 + 0.07456xCD34) = ACD34. Of the patients that received previous radiation therapy (9) or cyclophosphamide (2), plerixafor was utilized in 78% and 100%, respectively. Previous lenalidomide therapy was present in 50% of the patients and it did not correlate to any increase in plerixafor usage.
Adding plerixafor to G-CSF based upon a day 4 CD34+ count and collection yields is an effective strategy to mobilize CD34+ cells. Ninety-three percent of the of the patients were able to collect a minimum of 4.0×106 CD34+/kg cells and 99% collected > 2.0×106 CD34+/kg, in a median of two collections. Limitations to the study include a small sample size and an arbitrarily determined threshold to administer plerixafor. Also, the length of lenalidomide could not be retrospectively determined. A cost-based analysis is currently being performed to help determine the best day 4 CD34 cutoff for future studies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal