Abstract
Abstract 4377
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired complement-mediated hemolytic disorder. Eculizumab, a monoclonal antibody designed to inhibit terminal complement activation, reduces intravascular hemolysis while leading to improvements in anemia, fatigue, and quality of life as well as reductions in blood transfusions and thrombosis. This medication was approved by the Food and Drug Administration (FDA) in March 16, 2007. The intent of our study was to assess if the number of patients screened for PNH has increased after the approval of eculizumab by the FDA.
Retrospective single institution chart review over a period of 6 years from January 2004 to June 2010. Our study population comprised 174 patients screened for PNH by flow cytometry.
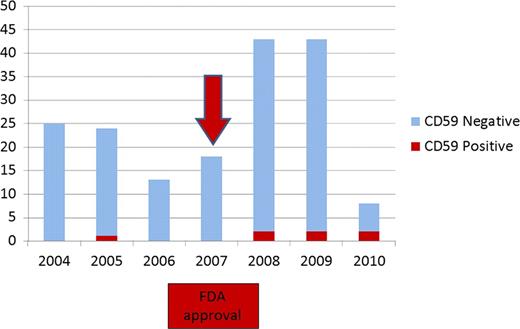
Between January 2004 and March 16, 2007 (39 months), a total of 68 cases were screened for PNH. On the other hand, between March 17, 2007 (date of FDA approval) and June 2010 (39 months), a total of 106 cases were screened for PNH with an increase in the frequency of testing by 55.8% (Figure 1). 1 out of 68 cases (1.5%) screened before March 16, 2007 were positive for PNH. 6 out of 106 cases (5.6%) screened after March 16, 2007 were positive for PNH (Table 1). 74 patients were male (42.5%) and 100 were female (57.5%). Average age at screening for PNH was 53.3 years. Screening was most commonly requested by Hematology (151 cases; 86.7%) followed by Internal medicine (5 cases), Hepatology (3 cases), Neurology (1 case) and Pulmonary (1 case). We found no information available in 13 cases regarding the location of the patient or the service that requested PNH screening. The clinical indications to screen for PNH were cytopenias (110 cases; 63.2%), thrombotic events (39 cases; 22.4%), or a combination of cytopenia plus a thrombotic event (9 cases; 5.2%). No indication for PNH screening was documented in 16 cases.
Our hypothesis is that an approval of a new medication can increase both, the awareness of a disease among physicians, and the frequency of ordering its diagnostic tests. The number of patients screened for PNH sharply increased after the approval of eculizumab by the FDA, confirming our hypothesis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal